The US National Institutes of Health (NIH) hosts regular COVID-19 Scientific Interest Group meetings. This week we highlight the COVID-19 SIG meeting that featured a talk by Dr Kizzmekia Corbett (NIH) on “SARS-CoV-2 mRNA Vaccine Development Enabled by Prototype Pathogen Preparedness.
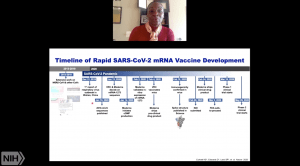
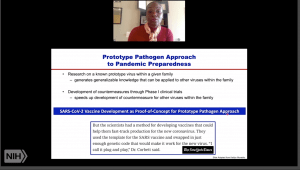 Dr Corbett began her talk emphasising that the potential for coronavirus to cause a global threat was known following the SARS (2002/3) and MERS (2012) epidemics. Thus, developing a fast, reliable and universal vaccine was highlighted as a potential solution to the impending threat. This could be achieved using a prototype pathogens approach to pandemic preparedness (see image), a process that requires an in-depth understanding of pathogen entry and development of immunity, to develop a vaccine against the virus.
Dr Corbett began her talk emphasising that the potential for coronavirus to cause a global threat was known following the SARS (2002/3) and MERS (2012) epidemics. Thus, developing a fast, reliable and universal vaccine was highlighted as a potential solution to the impending threat. This could be achieved using a prototype pathogens approach to pandemic preparedness (see image), a process that requires an in-depth understanding of pathogen entry and development of immunity, to develop a vaccine against the virus.
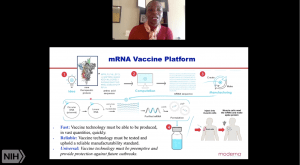 She then described research on MERS-CoV that the group undertook to develop a vaccine candidate against the virus and provided necessary groundwork that facilitated the development of mRNA-1273. Specifically, pre-clinical MERS-CoV DNA based vaccine studies identified the S1 region of the spike protein as a very immunogenic vaccine antigen. mRNA vaccine platform provides a fast, reliable and universal technology that enables rapid development of GMP ready vaccines. In collaboration with Moderna and other academic institutions, they performed a proof of concept study that used an mRNA vaccine platform to deliver MERS-spike protein. This study successfully demonstrated the utility of the vaccine strategy in inducing neutralising antibodies that protected mice from a lethal challenge (Also read: Corbett et al., 2020 Nature).
She then described research on MERS-CoV that the group undertook to develop a vaccine candidate against the virus and provided necessary groundwork that facilitated the development of mRNA-1273. Specifically, pre-clinical MERS-CoV DNA based vaccine studies identified the S1 region of the spike protein as a very immunogenic vaccine antigen. mRNA vaccine platform provides a fast, reliable and universal technology that enables rapid development of GMP ready vaccines. In collaboration with Moderna and other academic institutions, they performed a proof of concept study that used an mRNA vaccine platform to deliver MERS-spike protein. This study successfully demonstrated the utility of the vaccine strategy in inducing neutralising antibodies that protected mice from a lethal challenge (Also read: Corbett et al., 2020 Nature). 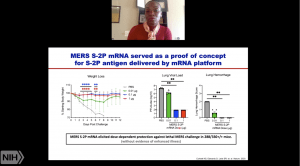 Thus, enabling rapid development of mRNA-1273 in 66 days (Corbett et al., 2020 Nature). She also described murine and non-human primate models that demonstrated the ability of mRNA-1273 to induce protective immunity in the lung and nose. If you have been following the COVID-19 vaccine pipeline, you would know that mRNA-1273 showed promising results in phase 1 clinical trials in both “young” and elderly adults, and is currently in phase 3 clinical testing.
Thus, enabling rapid development of mRNA-1273 in 66 days (Corbett et al., 2020 Nature). She also described murine and non-human primate models that demonstrated the ability of mRNA-1273 to induce protective immunity in the lung and nose. If you have been following the COVID-19 vaccine pipeline, you would know that mRNA-1273 showed promising results in phase 1 clinical trials in both “young” and elderly adults, and is currently in phase 3 clinical testing.
Read the following articles for more details about human trials.
- Safety and immunogenicity of the SARS-CoV-2 mRNA-1273 vaccine candidate in older age.
- mRNA Vaccine against SARS-CoV-2 induces robust Ab responses
She ended her talk highlighting that all these achievements were made possible via extensive collaborative projects with other research groups based at the University of Texas at Austin, The Scripps Research Institute, University of Washington, the University of North Carolina at Chapel Hill and many more.
Watch the full webinar at: NIH VideoCast and read the following article for more details. Corbett et al., 2020. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature
Summary by Cheleka AM Mpande