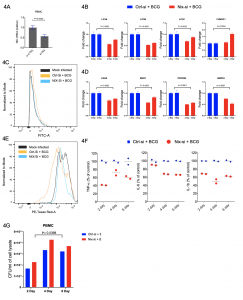
Glycolytic shift and metabolic rewirings are the least characterised mechanisms associated with f active tuberculosis infection. The host-pathogen interactions between Mycobacterium tuberculosis (Mtb) and alveolar macrophage disseminate immune regulatory signals to peripheral immune cells that govern disease outcomes. We show that chronic pulmonary tuberculosis infection primes circulatory monocytes into the pro-inflammatory M1 phenotype. RNASeq analysis of PBMCs from treatment naïve tuberculosis patients showed increased glycolysis and mitophagy pathways. Increased mitophagy corroborates with decreased oxidative phosphorylation. Selected genes (EIC2, HADH, LC3C, PFKFB3, NIX) qRT-PCR results from PBMCs of 22 healthy controls and 22 treatment naïve tuberculosis patients were in perfect agreement with RNASeq results. The closely related mycobacterium species M. bovis BCG also exert similar metabolic rewiring in PBMCs and human macrophage THP1 cells. Among key pathways having biological significance, mitophagy was a unique observation. Increased 2-NBDG uptake and decreased mito-tracker Red staining uptake indicate the substitution of OXPHOS by aerobic glycolysis. Increased consumption of 100% U-13C6-glucose and increased production of 100% U-13C6-lactate in BCG infected PBMCs culture supernatant, further support the glycolytic shift. Increased secretion of pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β) and increased ratio of genes NOS2/ARG1, CD86/CD206 in BCG infected macrophages indicated differentiation of M0 macrophages into M1 phenotype. NIX/BNIP3L expression abrogation, using gene-specific siRNAs, decreased expression of mitophagy, glycolysis pathways genes, and pro-inflammatory secretion cytokines. Both chronic tuberculosis infection or ex-vivo BCG infection of PBMCs and THP1, leading to increased expression of outer mitochondrial membrane receptor NIX, is essential for glycolytic shift and immune metabolic reprogramming of peripheral monocytes maturation and differentiation to M1 phenotype. In summary, NIX plays a central role in mitophagy mediated immune and metabolic reprogramming of peripheral immune cells during chronic pulmonary tuberculosis infection.
Journal Article: Mahla et al., Pre-Print. Protective Role of Mitophagy Associated Metabolic Reprogramming of Peripheral Macrophages During Tuberculosis.
Summary by Ranjeet Singh Mahla