A recent study highlights a key discovery that challenges previous assumptions about the role of an important immune protein, tumor necrosis factor (TNF), in infectious disease (Figure 1). This insight was made possible using a human cell culture model that mimics the lung’s essential immune cells, alveolar macrophages. The model is transforming the way scientists study immune responses in the lungs.
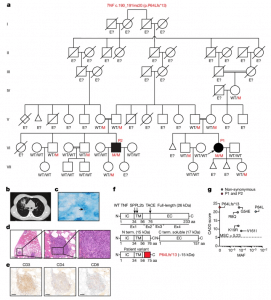
Figure 1: Identification of a biallelic TNF variant in two patients with pulmonary TB. a, Pedigree of two related kindreds showing familial segregation of the c.190_191ins20 (p.P64Lfs*13) TNF allele. Each generation is designated by a Roman numeral. Male and female individuals are represented by squares and circles, respectively. The filled boxes indicate individuals affected by TB and the crossed symbols indicate deceased individuals. ‘E?’ indicates an unknown TNF genotype. Red ‘M’ indicates the variant allele. The triangles indicate spontaneous abortion and the diamond indicates the death of an individual of unknown sex. b, Chest contrasted CT scan showing the pulmonary lesions of P1. c, Ziehl–Neelsen staining of a pulmonary biopsy specimen from P1 showing acid-fast bacilli (arrow). Data shown are representative of one independent experiment. d, Haematoxylin and eosin staining of a granuloma from P1 at different magnifications. Data shown are representative of one independent experiment. Scale bars, 2 mm, 200 µm and 50 µm (from left to right). e, Immunohistochemical staining for CD3, CD4 and CD8, indicating the presence of T cells within the granuloma of P1. Data shown are representative of one independent experiment. Scale bars, 200 µm. f, Schematic of the full-length and cleaved TNF proteins, with the intracellular (IC), transmembrane (TM) and extracellular (EC) domains indicated. The red part of the mutant TNF protein (P64Lfs*13) corresponds to the amino acids inserted due to the frameshift variant. aa, amino acids. N term. and C term., N-terminal and C-terminal fragments. g, The minor allele frequency (MAF; gnomAD, v.2.1.1) and combined annotation-dependent depletion (CADD; v.1.6) score for biallelic non-synonymous TNF variants reported in gnomAD v.2.1.1 or found in P1 and P2. The MSC is indicated by a dotted line.
Alveolar macrophages reside deep within the lungs, where they play a critical role in clearing pathogens like bacteria and viruses. However, obtaining these specialized cells for research traditionally requires invasive, time-consuming, and costly procedures. The researchers devised an innovative method to generate “alveolar macrophage-like” (AML) cells in the laboratory. These cells closely resemble the native alveolar macrophages, offering an easier, non-invasive alternative for research.
This model has already made significant contributions to global biomedical research. In the recent studies, AML cells were used alongside other cell-based models and extensive human genetic studies to investigate TNF’s function in tuberculosis (TB). The research offers a new perspective on TNF’s role in the immune system.
Traditionally, TNF has been considered essential for protecting against a broad range of infections. However, this study shows that while TNF is critical in defending against TB, it does not play the same role in other infectious diseases.
The AML model, combined with other experimental approaches, helped researchers uncover why TNF is so crucial in TB. TNF triggers the release of reactive oxygen species (ROS) within alveolar macrophages, which are key molecules that help eliminate Mycobacterium tuberculosis, the bacteria responsible for TB. In the absence of TNF, ROS production is impaired, allowing the bacteria to spread within the lungs.
The AML model offers significant advantages over previous methods, which required invasive lung washes to collect alveolar macrophages. Other macrophage models exist, but they often lack the unique features of alveolar macrophages. The AML model, derived from a simple blood draw, transforms blood monocytes into cells that closely mimic alveolar macrophages within a week. These cells are now being tested in more complex lung-on-chip models and with stem cells to generate even larger quantities for future studies.
Journal article: Arias, A.A., et al., 2024. Tuberculosis in otherwise healthy adults with inherited TNF deficiency. Nature.
Summary by Stefan Botha










