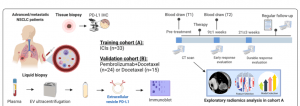
In a recent paper, de Miguel-Perez, et al., have described, validated and tested a blood test that, for the first time, can be used as a liquid biopsy to predict a patient’s response to cancer immunotherapy and whether it will be successful or not (Figure 1). This test also proves better for the patient and practitioners as opposed to the traditional invasive tumour biopsy procedure.
The test probes for the biomarker PD-L1. This protein is a target for immunotherapy and belongs to a group called checkpoint inhibitors (READ MORE). These inhibitors allow for the targeting and attack of cancer cells. In this present study, the researchers were able to show how this test is better for the prediction of immunotherapy responses and survival for patients with lung cancer than traditional methods probing for the same marker.
Extracellular vesicles (EV) are shed from tumour cells and carry a cargo of proteins, lipids, genetic material and more. For this test the specific focus of the test was the marker named EV PD-L1. This marker, due to there fluctuating levels during cancer, has become a marker of interest for the prediction of cancer progression and immunotherapy response.
For this study blood samples were collected from two cohorts of 33 and 24 patients with lung cancer (non-small cell) who were on immune-checkpoint inhibitor treatment at specific timepoints. As a control they included a group of patients on chemotherapy.
Journal article: de Miguel-Perez, D., et al., 2022. Extracellular vesicle PD-L1 dynamics predict durable response to immune-checkpoint inhibitors and survival in patients with non-small cell lung cancer. Journal of Experimental & Clinical Cancer Research.
Summary by Stefan Botha