Organ transplantation has long been one of medicine’s most significant achievements, providing life-saving treatments for patients with failing organs. However, one of the greatest ongoing challenges in transplantation is the immune system’s rejection of the new organ. The body’s immune system often identifies a transplanted organ as a foreign invader and launches an attack, leading to rejection. To prevent this, patients must take immunosuppressive medications for life, which dampen the immune response. Unfortunately, while these drugs help to prevent rejection, they also leave patients vulnerable to infections and reduce the effectiveness of vaccines.
In a new study, researchers are pioneering a novel approach that may one day eliminate the need for lifelong immunosuppression (Figure 1). They demonstrated a technique using nanoparticles to protect transplanted hearts in mice from immune rejection. The nanoparticles were designed to modify immune cells so that they would no longer attack the transplanted organ.
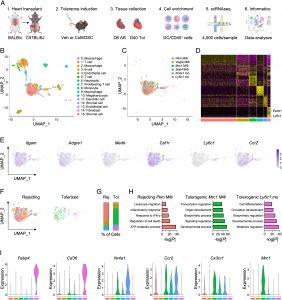
Single-cell RNA sequencing (scRNAseq) of cardiac allografts during acute rejection or tolerance reveals unique populations of tolerogenic macrophages and monocytes. (A) Experimental design for scRNAseq of cardiac allografts. Allografts were collected either 8 d after transplant in vehicle-treated mice for acute rejection or 40 d after transplant in mice treated with costimulatory blockade (CoB) and donor-specific cells (DSC) for tolerance. (B) Identification of 16 unique clusters by uniform manifold approximation and projection (UMAP) in combined conditions. (C) Identification of six unique clusters of macrophages and monocytes in combined conditions. (D) Heatmap of the 20 most differentially expressed genes within macrophage and monocyte clusters. (E) Feature plots representing single-cell expression of macrophage and monocyte marker genes. (F) Macrophage and monocyte clusters present during acute rejection or tolerance. (G) Proportion of macrophage and monocyte clusters during acute rejection or tolerance. (H) Pathway enrichment of differentially expressed genes in macrophage and monocyte clusters. Enrichment is expressed as the −log(P) and is adjusted for multiple comparisons. (I) Violin plots of genes expressed in macrophage and monocyte clusters.
This innovative approach could have profound implications not only for organ transplantation but also for other medical conditions involving immune rejection, such as diabetes, autoimmune disorders, and certain forms of cell therapy.
One of the key focuses of the research is to retrain the immune system to tolerate transplanted cells without shutting down the immune response entirely. This strategy hinges on modifying specific immune cells called myeloid cells, which play a pivotal role in recognizing and responding to foreign bodies. Myeloid cells, which include monocytes, are essential components of the immune system that can transform into inflammatory macrophages—cells tasked with attacking perceived threats.
A critical discovery was the role of a protein called HIF-2α in preventing immune rejection. This protein was found in the hearts of mice that successfully accepted transplants but was absent in those that rejected the organs. The researchers hypothesized that boosting levels of HIF-2α in myeloid cells could promote tolerance to the transplanted organ.
To test this, the team developed nanoparticles loaded with Roxadustat, a drug known to increase HIF-2α levels in monocytes. They targeted the spleen, a reservoir for these immune cells, with the nanoparticles. By modifying monocytes before they could turn into inflammatory macrophages, the researchers were able to significantly improve the acceptance of transplanted hearts in mice. Importantly, this approach allowed the immune system to continue functioning normally, without compromising the body’s ability to fight off infections.
This research offers new hope for the future of organ transplantation and immune-related therapies.
Journal article: DeBerge, M., et al., 2024. Hypoxia inducible factor 2α promotes tolerogenic macrophage development during cardiac transplantation through transcriptional regulation of colony stimulating factor 1 receptor. Proceedings of the National Academy of Sciences.
Summary by Stefan Botha










