In a recent paper, researchers have described how inhibiting or blocking calcium signals in immune cells can lead to a reduction in common asthma symptoms without effecting the immune response to flu viruses (Figure 1).
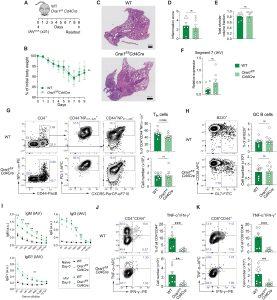
Figure 1: Deletion of Orai1 in T cells does not attenuate pulmonary inflammation and antibody production after infection with IAV. (A) Mouse model of x31 (H3N2) IAV infection. (B) Body weight of WT versus Orai1fl/flCd4Cre mice after infection with IAV, shown as weight normalized to day 0. (C) Representative hematoxylin and eosin (H&E) stains of lungs from WT and Orai1fl/flCd4Cre mice at day 9 after infection with x31 influenza A. Scale bar, 500 μm. (D) Inflammation score calculated from the lungs of WT and Orai1fl/flCd4Cre mice at day 9 following IAV infection. (E) Total alveolar volume fraction of lungs from WT and Orai1fl/flCd4Cre mice. (F) qRT-PCR analysis of IAV (segment 7) levels in total lung tissue from WT versus Orai1fl/flCd4Cre mice at day 9 after infection. (G) Representative flow cytometry plots of IAV-specific CXCR5+PD-1+ Tfh cells, their frequencies (%), and absolute numbers in mediastinal lymph nodes (mLNs) from WT and Orai1fl/flCd4Cre mice 9 days after IAV infection. (H) Representative flow cytometry plots of B220+CD38−GL7+ GC B cells, their frequencies (%), and absolute numbers in mLNs from WT and Orai1fl/flCd4Cre mice 9 days after IAV infection. FITC, fluorescein isothiocyanate. (I) IAV (x31)–specific IgM, IgG, and IgG1 levels in the serum of WT and Orai1fl/flCd4Cre mice before (day 0) and after (day 9) IAV infection. a.u., arbitrary units. (J and K) Analysis of TNF-α– and IFN-γ–producing CD4+CD44+ (J) and CD8+CD44+ (K) T cells from mLNs of WT and Orai1fl/flCd4Cre mice at day 9 after IAV infection. Representative flow cytometry plots, frequencies (%), and total numbers of T cells. Data in (A) to (K) are the means ± SEM of eight WT and eight Orai1fl/flCd4Cre mice and two independent experiments. Statistical analysis is performed by unpaired Student’s t test with the following significance levels: ***P < 0.001; **P < 0.01; *P < 0.05. ns, not significant.
The researchers knocked out the calcium release-activated calcium (CRAC) channel which is made up of ORAI1 protein. In a murine model, the knockout reduced asthmatic inflammation caused by dust mite faeces.
In the past, CRAC channels have also been shown to regulate the immune response to viruses and pathogens. They were able to show how one could inhibit these channels and reduce asthmatic inflammation without harming the immune response to viruses and infections. In addition, they tested the blocking of signals sent through the CRAC channel with CM4620, a new drug targeting the CRAC channel, and showed a suppression of Th2-driven airway inflammation. This drug is under development by the company CalciMedica.
The study highlighted a new class of drugs which target CRAC channels to combat allergic asthma without creating vulnerability to infections. This type of asthma is caused by increased type 2 (T2) inflammation, which involves a T cell subset called T helper (Th) 2 cells.
Journal article: Wang, Y.H., et al., 2022. Distinct roles of ORAI1 in T cell–mediated allergic airway inflammation and immunity to influenza A virus infection. Science Advances.
Summary by Stefan Botha










