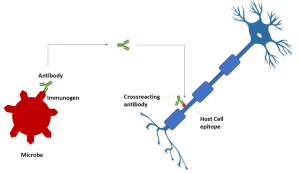
A simplified paradigm of molecular mimicry provoking the immune system to attack the nervous system.
Molecular mimicry drives the pathogenesis of autoimmune neuropathies such as Guillain-Barré syndrome and multiple sclerosis. Prototypically antibodies develop against microbial lipopolysaccharides in Guillain–Barré syndrome, a flaccid ascending paralytic peripheral neuropathy of human. But the structure of bacterial glycoconjugate antigens is similar to that of surface glycoconjugates of human peripheral nerve membrane. This results in the generation of antibodies against bacterial lipopolysaccharides in the aim of host protection against pathogens, which misleadingly react against the surface gangliosides of peripheral neurons. The body’s immunosurveillance turns into civil war out of an illusion. The anti-carbohydrate antibodies can be generated in a T-cell dependent or independent pathway (Kappler & Hennet, 2020). Recent studies compared SARS-CoV-2 peptides with human proteome to figure out the peptide-sequence similarity index (Marino Gammazza et al., 2020; Venkatakrishnan et al., 2020). Lucchese et al,2020 discovered the chaperones which are suspected to take part in molecular mimicry event as a consequence of infection of SARS-CoV-2. (Lucchese & Flöel, 2020). The research team further determined what immunological significance the shared motifs have using the Immune Epitope Database (IEDB), a repository of experimentally confirmed immune-positive epitopes. SARS-CoV-2 found to share EIPKEE and DKKKK hexapeptides with and HSP60 and HSP 90 (HSP90B and HSP90B2) respectively. Interestingly of patients with multiple sclerosis, the elevation of antibodies against HSP families in serum as well as cerebrospinal liquor is described. Future experimental studies are required to validate the HSP induced molecular mimicry in pathogenesis of autoimmune neurological sequelae of SARS-CoV-2.
Beguilingly we may make use of molecular mimicry in development of therapeutics for targeting Pathogen Associated Molecular Pattern (PAMP) too. For example, a recent revelation of structural masquerade of azithromycin (a SARS-CoV-2 treating drug) to sugar moiety of mono-sialic ganglioside GM1 (Fantini et al., 2020). This makes ganglioside-binding site of the spike protein of SARS-CoV-2 to interact with azithromycin. The shared binding domain of GM1 and azithromycin have a tripeptide consensus Q(134)-F(135)-N(137) of the spike protein’s tip. So, azithromycin competitively inhibits SARS-CoV-2 binding to membrane ganglioside of the host cell. Understanding of molecular mimicry in relation with SARS-CoV-2 can also lend credence in designing quality-assured vaccines.
Journal Article References:
- Fantini, J., Chahinian, H., & Yahi, N. (2020). Synergistic antiviral effect of hydroxychloroquine and azithromycin in combination against SARS-CoV-2: What molecular dynamics studies of virus-host interactions reveal. International Journal of Antimicrobial Agents, 56(2), 106020.
- Kappler, K., & Hennet, T. (2020). Emergence and significance of carbohydrate-specific antibodies. Genes and Immunity
- Lucchese, G., & Flöel, A. (2020). SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress and Chaperones
- Marino Gammazza, A., Légaré, S., Lo Bosco, G., et al., (2020). Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: possible role of molecular mimicry in COVID-19. Cell Stress and Chaperones, 1.
- Venkatakrishnan, A. J., Kayal, N., Anand, P., Badley, A. D., & Soundararajan, V. (2020). Multi-pronged human protein mimicry by SARS-CoV-2 reveals bifurcating potential for MHC detection and immune evasion. BioRxiv, 2020.06.19.161620.
Summary by Debprasad Dutta










