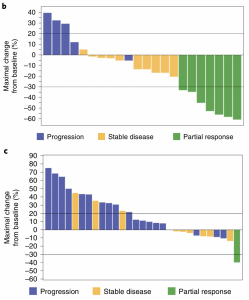
Waterfall plot analysis for the evaluable population showing the maximal percentage change in the sum of the longest diameters (mm) of target lesions as compared to baseline for patients treated with (b) BL-8040 and pembrolizumab combined with chemotherapy (fluorouracil, leucovorin and liposomal irinotecan) (mITT; N = 22) or (c) BL-8040 and pembrolizumab alone (mITT; N = 29). Blue, disease progression; yellow, stable disease; green, partial response. (Source: Bockorny et al., 2021)
Pancreatic cancer is one of the top 10 causes of cancer, with a global incidence of just over 450000 people in 2018 (Rawla et al., 2019). Unfortunately, despite advancements in diagnosis management of pancreatic cancer, the disease is only diagnosed during late stages of pathogenesis resulting in approximately 25% survival rate in the first year post-diagnosis. Pancreatic adenocarcinoma (PDAC) is the most common type of pancreatic cancer (85%). The development of PD-1 inhibitors for cancer immunotherapy brought some hope for treatment of PDAC, however effects were limited, suggesting the PDAC immunotherapy should likely to target multiple aspects of immunity. In line with this, researchers tested (phase 2 a clinical trial) the ability of combination therapy of pembrolizumab (PD-1 inhibitor) and BL-8040 (CXCR4 antagonist) with or without chemotherapy. Why did researchers choose this combination?
Recent evidence suggests that CXCR4 and its ligand, CXCL12, modulate the immune microen- vironment in pancreatic cancer preclinical models and, inhibition of the CXCR4–CXCL12 pathway enhanced T cell access to the tumor microenvironment (TME) and increased tumor sensitivity to anti-PD-1 ligand-1 (PD-L1) therapy in those models. (Source: Bockorny et al., 2021)
Bockorny et al., showed that treatment with pembrolizumab and BL-8040 alone did not result in a favourable outcome with the majority of individuals maintaining stable disease or developed progressive PDAC with only one individual experiencing a partial response. In this sub-cohort, BL-8040 did improve cytotoxic T cell infiltration to the tumour microenvironment (TME) and induced changes if the TME, despite resulting in a favourable outcome. Based on preclinical evidence from unpublished data which demonstrated that chemotherapy potentiates the anti-tumour effect pembrolizumab and BL-8040 improving the outcome of PDAC, researchers decided to test this combination therapy to improve the efficacy pembrolizumab and BL-8040 alone in PDAC patients. Of the 22 patients recruited, 17 individuals experienced a partial response (n=7) or stable diseases (n=10), thus demonstrating the superiority pembrolizumab, BL-8040 and chemotherapy compared to pembrolizumab and BL-8040 alone. This trial provided hope for an improved PDAC-specific immunotherapy in the near future and warranted the commission of phase 3 trial.
Journal Article: Bockorny et al., 2020. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nature Medicine
Summary by Cheleka AM Mpande










