A new study has shown that extracellular vesicles (EVs) play a crucial role in boosting T cell effector functions during viral infections (Figure 1). The study developed a method to analyse cell-bound phosphatidylserine+ (PS+) EVs and their target cells in vivo, demonstrating that EV+ cell abundance increases during viral infection and that EVs preferentially bind to activated, but not naive, CD8+ T cells.
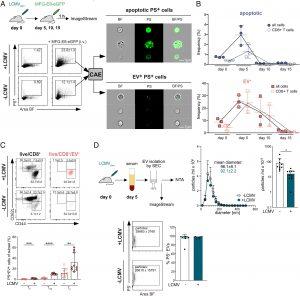
Figure 1: MFG-E8-eGFP detects PS+ apoptotic and PS+ EV-decorated cells in vivo. (A) Flow chart shows experimental setup for analysis of PS+ cells in mice. Splenocytes were analyzed by IFC using an ImageStream cytometer. Dot plots display the gating strategy of live/dead−PS+ cells from the spleens of noninfected and LCMVArm-infected mice. Area and aspect ratio of the bright field (BF) channel were used to identify single cells (SI Appendix, Fig. S1). Numbers in the gate indicate the mean percentages ±SD of PS+ cells from MFG-E8-eGFP-injected mice (n = 3) and control (PBS)-injected mice (n = 1). Only live cells were analyzed (SI Appendix, Fig. S1A) with the convolutional autoencoder (CAE) module as described previously (Kranich et al. 27). Representative images of bright field (BF), PS, and BF/PS overlay channels of PS− (SI Appendix, Fig. S1B) and PS+ cells are shown. (Scale bar: 7 µm.) Shown are representative results from two independent experiments. (B) Mice were infected with LCMVArm (n = 3 per timepoint), and frequencies of EV+ (red) and apoptotic (blue) total live cells and CD8+ T cells were determined on days 5, 10, and day 15 p.i. using the CAE. Gating see SI Appendix, Fig. S1C. Representative results from two independent experiments are shown. (C) Representative dot plots of different T cell subsets binding naturally occurring PS+EVs after i.v. administration of MFG-E8-eGFP are shown. Gates show total live CD8+ (Left, black) and live/CD8+/PS+EV+ (Right, red) CD62L+CD44– naive (TN), CD62L+CD44+ central memory (TCM), and CD62L−CD44+ effector (TE) T cells in spleens of LCMV-infected (Upper) and noninfected (Lower) mice. Values next to the gate indicate the frequencies ±SD of cells within the respective gate. Bar graphs visualize frequencies ±SD of TN, TCM, and TE CD8+ T cells that have bound PS+EVs. Bar graphs show the results from nine LCMV-infected and nine noninfected mice pooled from three independent experiments. (D) Analysis of serum EVs isolated from noninfected and LCMVArm-infected mice (day five p.i.). Upper panel shows nanoparticle tracker (NTA) analysis of EV fractions 2 to 7 isolated by size exclusion chromatography, n = 4, representative results from three independent experiments are shown. Bar graphs show particles/mL in noninfected and infected sera, n = 11, pooled results from three independent experiments are shown. Lower panel shows ImageStream analysis of serum EVs. Gating strategy, unstained, dye only, and detergent controls see SI Appendix, Fig. S1D. Dot plots show PS+ EVs present in sera of infected and noninfected mice. Mean number (±SD) of PS+ particles are indicated next to the gate. Bar graph shows percentage of PS+ EVs (n = 7 and 8 for noninfected and infected, respectively). Pooled results of two independent experiments are shown. Unpaired Student’s t test was used to determine statistical significance, with *P < 0.05, **P < 0.01, and ***P < 0.001.
Super resolution imaging revealed that PS+ EVs attach to clusters of CD8 molecules on the T cell surface. Furthermore, EV-binding induces antigen-specific TCR signalling and increased nuclear translocation of the transcription factor Nuclear factor of activated T-cells (NFATc1) in vivo. EV-decorated but not EV-free CD8+ T cells are enriched for gene signatures associated with T-cell receptor signalling, early effector differentiation, and proliferation. The study provides convincing evidence from in vivo studies that this interaction increases the expression of effector genes and proliferation of CD8+ T cells in LCMV-infected mice, thus demonstrating that PS+ EVs provide antigen-specific adjuvant effects to activated CD8+ T cells in vivo.
These findings open up new avenues for the development of EV-based immunotherapeutic strategies to boost T cell responses in viral infections.
Key takeaways:
1. EVs preferentially bind to activated, but not naive, CD8+ T cells.
2. EV-binding induces antigen (Ag)-specific TCR signaling and increased nuclear translocation of the transcription factor Nuclear factor of activated T-cells (NFATc1) in vivo.
3. EV-decorated but not EV-free CD8+ T cells are enriched for gene signatures associated with T-cell receptor signaling, early effector differentiation, and proliferation.
Counter arguments:
1. While some reports showed that EVs alone can activate T cells in vitro, other studies suggested that an indirect mode of action as DCs was required for the stimulation of T cells by EVs.
2. The precise mechanisms of T-cell stimulation by EVs are still unknown.
Journal article: Rausch, L., et al., 2023. Phosphatidylserine-positive extracellular vesicles boost effector CD8+ T cell responses during viral infection. Proceedings of the National Academy of Sciences.
Summary by Gaurang Telang










