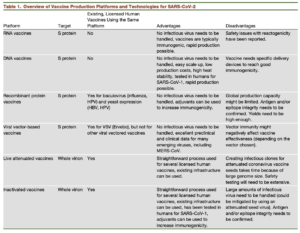
As the number of novel coronavirus infections rises daily across the globe, strategies for developing a safe and effective vaccine are rapidly moving forward. Recently published perspective by Amanat & Kramer highlights 6 main vaccine-platforms being investigated (Table).
Researchers from the Precision Vaccines Program (PVP) at Boston Children’s Hospital, Levy and Dowling, estimate that more than 24 COVID-19 vaccine candidates are in development globally. PVP are also designing a SARS-CoV-2 vaccine, however, they are building on earlier coronavirus vaccines, learning from earlier vaccines from prior coronavirus outbreaks and making them more effective. Multiple consideration go into designing vaccines, some of which include:
- Focusing on adjuvants: The current antigen used for vaccine development is the coronavirus spike protein, so named because it sits atop the spike of a coronavirus particle. This is the part of the virus that the immune system “remembers.” PVP’s strategy is to combine the coronavirus spike protein with adjuvants which are small molecules added to a vaccine to boost the recipient’s immune response.The team will test new adjuvants that has been discovered in the PVP’s National Institutes of Health-funded Adjuvant Discovery Program.
- Age specific:The PVP plans on testing a variety of adjuvants and adjuvant combinations in human white blood cells sourced from older people. Researchers will then study the adjuvant-induced immune responses.
- Testing in mice: The group is also studying coronavirus immune responses in a living animal model. The first mice have already been inoculated with a similar coronavirus spike protein derived from the SARS-2003 coronavirus with or without a lead adjuvant combination to get an early read on measuring an antibody response.
Some of the vaccine strategies currently in the clinical phase of development are:
- mRNA-1273 vaccine (US): co-developed by Moderna and Vaccine Research Centre based at the US-National Institutes of Health is the only vaccine of its kind in the clinal phase of vaccine development. “mRNA-1273 is a novel lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine that encodes for a full-length, prefusion stabilized spike (S) protein of SARS-CoV-2. ”
- INO-4800 (US): CEPI selected the Inovio Pharmaceuticals DNA vaccine platform to develop a vaccine against COVID-19. “This is an open-label trial to evaluate the safety, tolerability and immunological profile of INO-4800 administered by intradermal (ID) injection followed by electroporation (EP) using CELLECTRA® 2000 device in healthy adult volunteers.”
- Covid-19 aAPC Vaccine (China): This trial proposes to develop universal vaccine and test innovative Covid-19 minigenes engineered based on multiple viral genes, using an efficient lentiviral vector system (NHP/TYF) to express viral proteins and immune modulatory genes to modify artificial antigen presenting cells (aAPC) and to activate T cells. In this study, the safety and immune reactivity of this aAPC vaccine will be investigated. (Prevention and treatment of COVID-19 vaccine.)
- Covid-19 Synthetic Minigene Vaccine (China): Infection of Covid-19 is mediated through binding of the Spike protein to the ACEII receptor, and the viral replication depends on molecular mechanisms of all of these viral proteins. This trial proposes to develop and test innovative Covid-19 minigenes engineered based on multiple viral genes, using an efficient lentiviral vector system (NHP/TYF) to express viral proteins and immune modulatory genes to modify dendritic cells (DCs) and to activate T cells. In this study, the safety and efficacy of this LV vaccine (LV-SMENP) will be investigated.
- CTCOVID-19 (not recruiting, China): The investigators intent to evaluate the safety, reactogenicity and immunogenicity of Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector).
- COV001 (UK): A phase I/II single-blinded, randomised, placebo controlled, multi-centre study to determine efficacy, safety and immunogenicity of the candidate Coronavirus Disease (COVID-19) vaccine ChAdOx1 nCoV-19 in UK healthy adult volunteers aged 18-55 years. The vaccine will be administered intramuscularly (IM).
References:
- Alice McCarthy,. Boston Children’s (March 11, 2020). This article is the latest in Harvard Medical School’s continuing coverage of medicine, biomedical research, medical education and policy related to the SARS-CoV-2 pandemic and the disease COVID-19.
Article by Naffesa Al Sheik and Cheleka AM Mpande