Rheumatoid arthritis (RA), a chronic inflammatory joint disorder and remains a challenging condition to treat effectively. Despite advancements in therapeutic options, many patients fail to achieve remission due to the trial-and-error approach clinicians often take when prescribing treatments. A recent study has introduced a breakthrough method in precision medicine that could revolutionize treatment selection for RA and other autoimmune diseases (Figure 1).
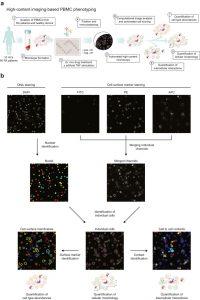
Figure: 1 Ex vivo profiling of PBMCs. (a) Experimental setup. PBMCs from patients with RA and healthy controls (HCs) were isolated by density gradient centrifugation and incubated for 24 h with immunomodulatory drugs and controls. Cells were fixed and stained without perturbing the monolayer culture and imaged. Automated image analysis was performed and information about cellular abundance, morphology and intercellular interaction was retrieved. (b) Computational image analysis. Nuclear staining was performed with DAPI and cell surfaces were labelled with surface marker-specific fluorescently-labelled antibodies. Illumination correction was applied to surface marker images and images were then merged. Cells were identified with nuclei as seeds based on the merged surface marker images and cell surface membranes were identified by shrinking cells by 3 pixels. Cell surface marker intensities were quantified on native images for cell type identification, cellular morphology was measured on individual cells and cell-to-cell contacts were quantified based on touching neighbouring cells. DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; PE, phycoerythrin; APC, allophycocyanin.
RA disproportionately affects women and is characterized by inflammation, joint pain, and damage that can severely impact quality of life. While various drugs target the immune system to reduce inflammation, a lack of reliable tools to predict patient responses has left many relying on an inefficient process of testing one drug after another. Current biomarkers offer limited predictive value, are not widely available for routine use, or involve invasive procedures, underscoring the urgent need for a more effective solution.
The research team developed and tested an innovative microscopy-based technology called Pharmacoscopy. This cutting-edge method allows for the direct observation of drug effects on individual immune cells, generating vast amounts of data in a fully automated manner. Unlike traditional molecular biology approaches, Pharmacoscopy does not require a detailed understanding of the underlying molecular mechanisms, streamlining the process of evaluating drug efficacy.
The method combines imaging technology with an ex vivo stimulation process, in which immune cells are extracted from patient blood samples and treated with RA medications outside the body. By analysing how these drugs influence immune cell behaviour under various conditions, researchers identified unique cellular phenotypes that correlate with disease activity and therapeutic response.
These cellular phenotypes have the potential to predict the success of different treatments for individual patients, offering a glimpse into a future where therapy can be tailored based on a simple blood sample. This approach moves away from the current trial-and-error model, providing a data-driven pathway to selecting the most effective treatment.
The findings also highlight the potential of this technology beyond RA, offering a framework for precision medicine in a range of autoimmune and inflammatory diseases. By systematically analysing drug effects on individual cells, researchers can gain valuable insights into the mechanisms of action for therapies, potentially transforming the way these diseases are managed.
Journal article: Kartnig, F., et al, 2024. Ex vivo imaging-based high content phenotyping of patients with rheumatoid arthritis, eBioMedicine.
Summary by Stefan Botha










