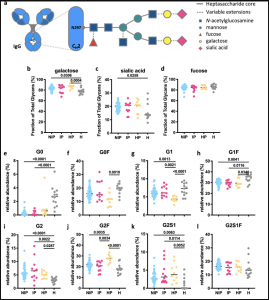
Glycan profiles of IgG Abs in pregnant women with malaria. (a) Schematic representation of N-linked glycan composition of human IgG Abs. The glycans are attached to asparagine (N) at position 297 in the CH2 domain of IgG and have a biantennary heptasaccharide core (solid line) and variable extensions (dash line), such as fucose, galactose and/or sialic acid. Relative abundance of specific types of N-linked glycan structures of purified IgG Abs from non-infected pregnant women (NIP; N = 41; blue), pregnant women with malaria infection (IP; N = 11; pink), malaria-naïve healthy pregnant women (HP; N = 10; yellow) and uninfected healthy non-pregnant women (H; N = 13; grey) were profiled. % of Fc glycans with the presence of (b) galactose (monogalactosylated or digalactosylated), (c) sialic acid and (d) fucose. (e–l) The relative prevalence of several major glycan structures (G0 agalactosylated, G1 monogalactosylated, G2 digalactosylated, F fucosylated, S1 sialylated). Statistical comparison between groups was performed using a Kruskal–Wallis test corrected for multiple comparisons using Dunn’s multiple comparison method (p-values are shown on graphs). (Source: Damelang et al., 2021)
Pregnant women are more susceptible to malaria due to immunological changes and the characteristics of the Plasmodium falciparum (P. falciparum) parasite. The variant surface antigen VAR2CSA, which is expressed on P. falciparum infected erythrocytes, prevents splenic clearance of these erythrocytes. This leads to inflammation and localized endothelial dysfunction of the placenta. Women with high plasma levels of IgG antibodies recognizing VAR2CSA have a decreased risk of delivering low-birthweight babies. Natural killer (NK) cells, which can mediate antibody-dependent cellular cytotoxicity (ADCC) upon engagement of their receptors, reportedly killed infected erythrocytes and can inhibit P. falciparum growth. Post-translational modifications of glycans on the Fc domain of the antibody are known to regulate effector functions such as ADCC.
Damelang et al. investigated the ability of IgG antibodies, specific to DBL2 and DBL3 (two VAR2CSA domains), of pregnant women from a malaria-endemic area to activate human primary NK cells from malaria-naïve donors. This is of special interest since the two leading placental malaria candidates PRIMVAC and PAMVAC both include DBL2 domains. To this end, the authors collected plasma samples from pregnant women in Madang Province, Papua New Guinea and determined malaria infection status. As controls served malaria-naïve healthy donors from Melbourne, Australia, to avoid skewing of negative responses by previous exposure to malaria. Antibody-dependent NK cell activation was measured via flow cytometry and IgG glycosylation by microchip capillary electrophoresis with laser-induced fluorescence.
The authors found that primary human NK cells are activated by DBL2- or DBL3-specific IgG antibodies from pregnant women with malaria, which was largely associated with the upregulation of CD107a (surrogate marker for ADCC activity). Elevated anti-inflammatory total IgG glycosylation patterns were observed in pregnant women, although there was no significant difference between malaria-infected and malaria-naïve women. This suggest that the IgG glycosylation profile may be more influenced by the pregnancy than by malaria infection. However, it cannot be excluded that malaria-specific antibody glycosylation patterns are associated with clinically relevant outcomes in placental malaria and further research is needed.
Journal article: Damelang et al., 2021. Antibody mediated activation of natural killer cells in malaria exposed pregnant women. Scientific Reports
Article by Jasmin Knopf










