Alzheimer’s disease (AD) is a devastating disease. An estimated 6.7 million Americans age 65 and older are living with AD today. This number could grow to 13.8 million by 2060. While differences in memory-related brain circuitry based on biological sex are well-documented, the drivers of sex-specific aging and AD risk remain unclear. A recent study leveraging over 50 years of longitudinal data, provides new insights into how maternal immune activity during pregnancy shapes memory circuitry and function in offspring, with distinct patterns for males and females (Figure 1). The findings could advance understanding of Alzheimer’s origins and its lifelong development.
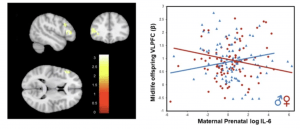
Figure 1: Sex differences in impact of maternal prenatal IL-6 on memory circuitry activity. Image illustrates significant sex effects on ventral lateral prefrontal cortex (VLPFC) during an fMRI verbal encoding task associated with high maternal prenatal IL-6 serum levels. In generalized linear model (GLM) analyses using generalized estimating equations (GEE) adjusting for age, parental SES, maternal race, and intrafamilial correlations between siblings, significant interactions of sex with prenatal exposure occurred across the VLPFC (Dorsal: pFDR = 0.03; Mid: pFDR = 0.03; Ventral: pFDR = 0.03).
The study analysed data from a cohort tracking individuals born from nearly 18,000 pregnancies between 1959 and 1966. Researchers focused on 204 participants exposed or not exposed to elevated maternal immune markers—such as cytokines IL-6 and TNF-a—during pregnancy. Functional brain imaging examined the impact of these early exposures on memory-related brain regions dense in cytokine and sex hormone receptors, which exhibit sex-specific development starting in fetal stages.
Findings revealed that elevated maternal immune markers were associated with sex-dependent adverse effects on offspring memory circuitry and cognitive function. In postmenopausal women, these effects were pronounced, with higher markers of a proinflammatory state observed in midlife. Notably, these immune-driven effects on memory function were evident as early as age seven, suggesting a critical link between prenatal exposures and lifelong brain health.
The study highlights the potential role of heightened maternal immune activity in predisposing offspring to heightened immune and stress sensitivity. These findings highlight the importance of understanding the interplay between early immune exposure and subsequent brain development in shaping aging-related memory disorders.
This study marks a significant step toward understanding how fetal origins influence lifelong brain health. By integrating knowledge of sex-specific brain development and its interaction with prenatal factors, researchers hope to develop strategies that prevent or delay Alzheimer’s disease, offering new avenues for early intervention and tailored treatments.
Journal article: Goldstein, J.M., et al. 2024. Prenatal immune origins of brain aging differ by sex. Molecular Psychiatry.
Summary by Stefan Botha










