A recent study published in Nature examined the role of tissue-resident memory CD8+ T cells (TRM) in preventing respiratory virus transmission, focusing on their ability to curb virus spread in the absence of antibodies. Using a mouse model of natural parainfluenza transmission, the researchers demonstrated that respiratory TRM could effectively reduce viral transmission and limit infection rates. Unlike traditional antibody-based immunity, which respiratory viruses can evade through mutation, TRM remained effective due to their conserved viral recognition and rapid response at the site of infection.
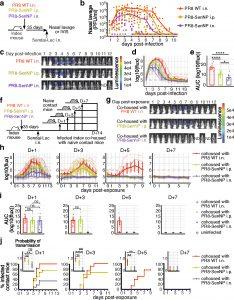
Figure 1: (a) Schematic of experiment to assess Sendai viral titers in the nasal cavity of immunized mice and investigate immunization route on protection from direct infection. (b) Sendai virus titers in the nasal cavity of immunized mice (n=10 for PR8 WT n=10 for PR8-SenNP i.p., n=9 for PR8-SenNP i.n.). c) Representative in vivo imaging (IVIS) images of immunized mice following direct infection with Sendai-Luc. (d) Bioluminescence curves over the course of infection (n=10 per group). (e) Area under curve (AUC) of bioluminescence signal over the course of infection (n=10 for each experimental group). (f) Schematic of experiment to investigate transmission from immunized index mice to naïve contact mice at indicated time points. (g) Example images of contact mice where index mice were added 3 days post infection. (h) Bioluminescence curves of contact mice following co-housing on day 1 (n=12 per group), day 3 (n=12 per group), day 5 (n=8 per group), and day 7 (n=8 per group) post infection of index mice. (i) AUC of bioluminescence of contact mice that become infected following co-housing with an infected index mouse. Grey circles represent uninfected mice. (j) Percent infected contact mice following co-housing with an infected index mouse and the probability of transmission (inset). Dots represent proportion of contact mice that become infected and error bars represent 95% binomial confidence intervals. All data are representative of 2 combined experiments with similar results. For bioluminescence curves (d, h), solid dark lines represent means, solid pale lines represent individual mice, dashed grey line represents background bioluminescence, and dashed red line represents the limit of infection. Solid lines (b, e, i, j) and error bars (d, e, h, i) represent mean with 95% confidence interval. Statistical significance was determined using a two-sided Mann Whitney test. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001, ns: non-significant.
The researchers immunized mice intranasal with vectors expressing Sendai virus CD8 T cell epitopes. Mice with pre-existing TRM in the respiratory tract showed a significantly reduced viral burden, shorter infection duration, and less transmission when exposed to infection. TRM functioned by producing antiviral cytokines, particularly IFN-γ, which altered the transcriptional profile of respiratory epithelial cells, inducing an antiviral state. Importantly, these TRM cells reduced viral replication, limiting not only individual infection severity but also the likelihood of spreading the virus to others.
The study emphasized the potential of T cell-based vaccines in providing durable, broad-spectrum immunity against respiratory viruses such as influenza and SARS-CoV-2. Unlike antibody-mediated vaccines, TRM-targeted vaccines might offer an alternative strategy, especially in populations where antibody escape variants arise. The findings suggest that TRM can complement existing vaccine strategies by reducing transmission risk and enhancing herd immunity.
This research highlights the importance of focusing on cellular immunity in the fight against respiratory infections, offering a promising avenue for developing next-generation vaccines.
Journal Article: Uddbäck, Ida, et al. “Prevention of Respiratory Virus Transmission by Resident Memory CD8+ T Cells.” Nature.
Summary by Faith Oluwamakinde










