Scientists have uncovered a critical missing piece in the puzzle of psoriasis, a chronic inflammatory skin disease. A new study identifies a specific gene mutation responsible for the development of psoriasis. More importantly, the research sheds light on why some patients with psoriasis progress to psoriatic arthritis, a condition affecting both the skin and joints (Figure 1).
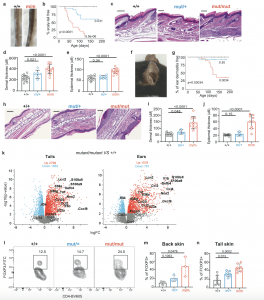
Figure 1: Overactive IKK2 leads to psoriasis. Representative images (a) and Kaplan-Meier plots of cumulative incidence (b) of tail pathology in Ikbkb+/+ (n = 13), Ikbkbmut/+ (n = 19) and Ikbkbmut/mut (n = 15) mice. Tail skin pathology revealed by representative images of skin sections stained with H&E (scale bar, 200 μm) (c), and summaries of dermal (d) and epidermal thicknesses (e). Ikbkb+/+, n = 15; Ikbkbmut/+, n = 7, Ikbkbmut/mut, n = 12. Ear skin pathology revealed by a representative image from an Ikbkbmut/mut mouse (f), Kaplan-Meier plots of cumulative incidence of dermatitis by genotype (g) (n = 13-18/genotype), representative images of sections of ear skin stained with H&E (scale bar, 100 μm) (h), and summaries of dermal (i) and epidermal thicknesses (j). Ikbkb+/+, n = 15; Ikbkbmut/+, n = 4; Ikbkbmut/mut, n = 9. k Volcano plot depicting differentially expressed genes in Ikbkbmut/mut and Ikbkb+/+ mice from tail and ear skin (n = 3/genotype). Red dots represent genes highly expressed in mutants relative to wild type while blue dots represent genes lowly expressed in mutants relative to wild type. Gene expression was normalized using trimmed mean of M values and differentially expressed genes were identified with a Benjamini–Hochberg adjusted p value < 0.05. Flow cytometric analysis of Foxp3 expression by CD4+ T cells from skin for indicated Ikbkb genotypes, with representative plots (l), enumeration of Tregs as a proportion of CD4+ T cells from back skin (n = 3/genotype) (m) and tail skin (n) (n = 7/genotype). Summary graphs are presented as mean +/− s.d. Kaplan-Meier curves: log-rank (Mantel-Cox) test; Frequency histograms, one-way ANOVA with Bonferroni’s multiple comparison test.
The study pinpoints a mutated gene called IKBKB as the culprit behind psoriasis. If someone has two copies of this mutated gene, they are more likely to develop the condition. This finding offers a significant step forward in understanding the root cause of psoriasis.
The research goes a step further, explaining why some psoriasis cases progress to psoriatic arthritis. When researchers examined mice with the IKBKB mutation, they discovered that the mutation disrupts the function of regulatory T cells, a key component of the immune system. Healthy regulatory T cells act as “gatekeepers,” keeping the immune system in check. However, the mutation throws a wrench into this process, causing the T cells to contribute to inflammation and promote the development of psoriatic arthritis.
This research holds promise for improving the lives of millions of people living with psoriasis and psoriatic arthritis. By understanding the role of the IKBKB gene, researchers are paving the way for more accurate diagnosis and potentially even the development of new treatments. Ultimately, the hope is to find a cure for these conditions, which can have a significant impact on a person’s quality of life, often carrying a social stigma.
Psoriasis and psoriatic arthritis are currently incurable, though treatments can manage the symptoms. This new understanding of the IKBKB gene mutation offers a beacon of hope for the future. By unraveling the mechanisms behind these diseases, scientists are one step closer to developing a cure that could provide lasting relief for patients.
Journal article: Cardinez, C., et al., 2024. IKK2 controls the inflammatory potential of tissue-resident regulatory T cells in a murine gain of function model. Nature Communications, 2024.
Summary by Stefan Botha










