In a recent paper, researchers have found that rebounding to COVID-19 is not likely to be caused by an inefficient or impaired immune response (Figure 1). Looking at 8 patients who have taken nirmatrelvir/ritonavir, they aimed to describe the immunological characteristics of these patients during COVID-19 rebound. Rebound can be defined as the recurrence of COVID-19 symptoms and/or a new positive viral test after having tested negative.
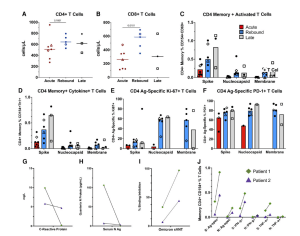
Figure 1: Comparison of CD4+ T-cell responses across the groups and longitudinal immune responses of the 2 patients with sampling at acute and rebound time points. Absolute T-cell counts compared across groups (A, B). Lines represent median values and points represent individual results. The 2 longitudinal patients are identified by an open triangle and open diamond. The empty square represents the coronavirus disease 2019 rebound patient who did not receive nirmatrelvir-ritonavir. T-cell subset flow cytometry data not available for longitudinal patient 2 (triangle) at rebound time point. T-cell stimulations were performed with peptide pools corresponding to spike, nucleocapsid, and membrane proteins as listed on the x-axis. Bars represent medians and groups are defined as acute, rebound, and late presentations. Severe acute respiratory syndrome coronavirus 2–specific CD4 T-cell responses are highlighted by memory (C), cytokine-producing (CD154 + IFN-γ+, CD154 + TNF-α+ or CD154 + IL-2+) (D), activated (CD154 + CD69+) (E), or Ag-specific proliferating (Ki-67+) and activated (PD-1+) T cells (F). For phenotyping of Ki-67 + and PD-1+ cells, a threshold of at least 20 events and a 2-fold increase over unstimulated cells was used, and samples were excluded if they did not meet these thresholds (E, F). Serum N Ag and C-reactive protein trends from the 2 longitudinal patients (G, H). T-cell responses and neutralizing antibodies from the acute and rebound presentation for 2 patients with longitudinal samples (I, J). T-cell responses are from S and N stimulations. Ag-specific CD4 T cells defined by (CD154 + CD69+, CD154 + IFN-γ+ and CD154 + TNF-α+), and neutralizing antibodies represented by percent binding inhibition on the sVNT. Abbreviations: Ag, antigen; S, spike; N, nucleocapsid; spec, specific; sVNT, surrogate virus neutralization test.
The researchers tested whether the five-day course of Paxlovid (nirmatrelvir/ritonavir) was efficient for the body to develop a sufficient immune response to SARs-CoV-2. They found no evidence of any genetic mutations that may lead to immunity of SARS-CoV-2 to Paxlovid and there was no delay in immunity development in the patients experiencing rebound following Paxlovid administration. Investigators detected robust SARS-CoV-2 T-cell responses in rebound patients.
In short, the researchers were able to find that the level of T-cell responses was increased in patients suffering from COVID-19 rebound than in patients who were experiencing early and acute COVID-19 infection without rebound.
The findings of this study were able to shed light on COVID-19 rebound, highlighting that rebound symptoms may be seen due to an immune response against residuals viral RNA and not an impaired immunity to the virus which allows for disease progression. The study would need to increase in sample size to validate the authors findings.
Journal article: Epling, B.P., et al., 2022. Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment. Clinical Infectious Diseases.
Summary by Stefan Botha










