Glioblastoma is the most common and aggressive form of malignant brain tumour in adults, with no current treatments capable of eradicating it permanently. Its resistance to therapy is due to the tumour’s cellular diversity and a supportive microenvironment that protects it from attack. Researchers have now developed an innovative immunotherapy that not only targets the tumour but also transforms its surrounding environment into an ally against the cancer (Figure 1).
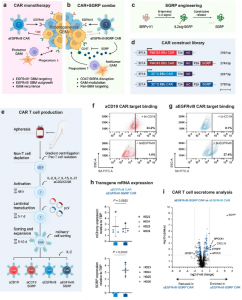
Figure 1: Anti-EGFRvIII-SGRP CAR T cells constitutively secrete SGRP, enabling CD47-SIRPα axis disruption. a Mechanism of action of conventional aEGFRvIII CAR T cell monotherapy in GBM. b Proposed aEGFRvIII-SGRP CAR T cell combination therapy whereby SGRP-mediated CD47 blockade induces phagocytic modulation of GAMs in the context of EGFRvIII-heterogenous GBM and its immunosuppressive iTME. c Outline of the SGRP engineering strategy, including specific AA substitutions to the endogenous human SIRPγ-V1 sequence and addition of an N-terminal IL-2 signal sequence (IL2sig) leading to constitutive SGRP secretion. d Polycistronic lentiviral constructs encoding mCherry (mC)-labeled aCD19 CAR or aEGFRvIII CAR under the control of EF1A promoter ± SGRP secretion. e Workflow of CAR T cell production applied throughout the study. a–e Created in BioRender. Hutter, G. (2022) BioRender.com/u48r093. Representative plots of CAR:target protein binding by aCD19 CAR T cells (f) or aEGFRvIII CAR T cells (g) to CAR-bound biotinylated (bt)-CD19 (top plots) or bt-EGFRvIII (bottom plots); n = 2 healthy donors (HDs) assessed per CAR. h TATA-box binding protein (TBP)-normalized expression of mCherry and SGRP detected by real-time quantitative PCR (RT-qPCR) in aEGFRvIII CAR or aEGFRvIII-SGRP CAR T cells, showing mCherry expression in CARs transduced with either construct and SGRP expression specifically in aEGFRvIII-SGRP CARs; n = 4 HDs. Data are presented as scatter plots with mean values ± SD. Statistical differences were assessed by two-sided unpaired t tests with Welch’s correction. i Differentially secreted proteins in aEGFRvIII-SGRP CAR- vs aEGFRvIII CAR-conditioned media, highlighting the presence of SGRP exclusively in aEGFRvIII-SGRP CAR; n = 2 HDs. Mean SGRP expression: -log10qValue/log2foldchange=8.60/6.65. Source data are provided as a Source Data file. Source data for (i) are provided as Supplementary Data 1.
This new approach builds on the success of CAR T-cell therapy, a promising immunotherapy technique. CAR T-cells are engineered from a patient’s own T-cells in the laboratory, where they are equipped with a chimeric antigen receptor (CAR) to recognize and destroy cancer cells. These reprogrammed cells are then reintroduced into the patient’s body, where they act as precise hunters against cancer. While highly effective in treating certain leukaemia’s, CAR T-cell therapy has faced challenges with solid tumours, including glioblastoma.
Glioblastomas present multiple obstacles. First, CAR T-cells struggle to penetrate the tumour. Second, not all glioblastoma cells display the specific structures that CAR T-cells can recognize. Finally, the tumour’s microenvironment actively suppresses immune responses. This is especially true in the brain, where T-cells are typically absent, and the environment is inherently hostile to immune cells.
The team developed an advanced version of CAR T-cell therapy specifically designed to overcome glioblastoma’s defences. Their approach involves collecting a patient’s T-cells during the recovery period following surgery to remove the tumour. These T-cells are engineered in the lab to target glioblastoma cells and are then directly injected into the regrowing tumour, by passing the barrier of accessing the solid tumour.
In addition to targeting cancer cells, the modified CAR T-cells include a blueprint for a molecule that disrupts the tumour’s ability to manipulate surrounding immune cells. Glioblastomas typically hijack microglia and macrophages, key immune cells, turning them into allies that suppress the body’s immune response. By blocking the tumour’s signals, the engineered CAR T-cells reverse this manipulation, converting these “traitor” immune cells back into defenders. This allows microglia and macrophages to support the CAR T-cells in their attack, even against glioblastoma cells that lack the specific target structure.
This novel immunotherapy offers a promising step forward in treating glioblastoma, providing hope for patients battling this formidable disease. If successful in clinical trials, it could also pave the way for similar approaches to combat other solid tumours and resistant cancers.
Journal article: Tomás A. Martins, T.M., et al., 2024. Enhancing anti-EGFRvIII CAR T cell therapy against glioblastoma with a paracrine SIRPγ-derived CD47 blocker. Nature Communications.
Summary by Stefan Botha










