Resident memory T (TRM) cells represent a critical component of the immune system’s defence against cancer, offering a localised and long-lasting immune response within tissues. The article “Resident Memory T Cells and Cancer Immunity” thoroughly examines the role of TRM cells in cancer immunology, focusing on their unique functions, mechanisms of action, and potential as therapeutic targets in cancer treatment (Figure 1).
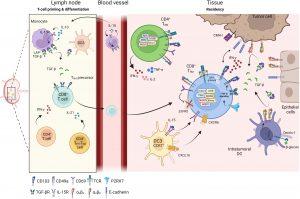
Figure 1: Differentiation of TRM in tissue. Priming and differentiation of naïve CD8+ T cells take place in the lymph node in contact with monocytes or dendritic cells (DC3) which promote the generation of CD8+ TRM precursors. Through cytokines secretion such as IFN-γ or IL-21, CD4+ T cells help CD8+ T cells to be fully functional and influence the TRM differentiation. Primed CD8+ TRM precursor cells reach the inflamed sites where they differentiate into CD8+ TRM. Within the tissue, several cellular components regulate and maintain TRM cells: DC3 participate in positioning CD8+ TRM cells in an immune niche and promote their survival, DC infiltrating tumors, tumor cells or epithelial cells express integrin αvβ6 and αvβ8 and transform LAP-TGF-β to active TGF-β. Active TGF-β maintains tissue residence of TRM cells and initiates CD103 signaling leading to functional and cytotoxic TRM cells. CD4+ TRM cells have recently been identified and are also present in peripheral tissues and solid tumors where they secrete inflammatory cytokines such as IFN-γ, TNF-α or IL-2.
TRM cells are a specialised subset of memory T cells that reside permanently in tissues rather than circulating in the blood. These cells are strategically positioned at common sites of infection or tumorigenesis, such as the skin, lungs, and gastrointestinal tract, where they provide rapid and effective immune responses upon re-exposure to antigens. The article details how TRM cells differ from other memory T cells, particularly in their ability to respond immediately to local threats without needing to recruit additional immune cells from the bloodstream.
One of the central functions of TRM cells in cancer immunity is their ability to recognize and eliminate tumour cells directly within the tissue. The article discusses the mechanisms by which TRM cells exert their cytotoxic effects, including the secretion of perforin and granzyme B, which induce apoptosis in target cells. This direct killing ability is especially crucial in the early stages of tumour development, where the presence of TRM cells can prevent the establishment and growth of tumours by eliminating nascent cancer cells.
The formation and maintenance of TRM cells within tissues are governed by specific cytokines and transcription factors. The article highlights the roles of interleukin-15 (IL-15) and transforming growth factor-beta (TGF-β) in promoting TRM cell development and retention. These cytokines induce the expression of transcription factors such as Hobit and Blimp-1, which are essential for TRM cell differentiation and survival in non-lymphoid tissues. The tissue microenvironment, which includes interactions with resident stromal and immune cells, also plays a crucial role in maintaining the TRM cell phenotype and function.
Another significant aspect covered in the article is the role of TRM cells in tumour immunosurveillance and control. TRM cells are positioned to detect early signs of tumorigenesis, initiating a robust immune response that includes the recruitment of additional cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells to the tumour site. The article discusses how this coordinated immune response is vital for controlling tumour growth and preventing metastasis. Additionally, the article explores the interactions between TRM cells and other immune cells in the tumour microenvironment, including their ability to modulate the activity of dendritic cells and macrophages, thereby influencing the broader immune response to tumours.
The potential of TRM cells as therapeutic targets in cancer immunotherapy is a major focus of the article. Given their ability to provide long-term immunity within tissues, TRM cells offer a promising avenue for cancer treatment. The article reviews various strategies to enhance TRM cell function in cancer therapy, such as vaccines designed to induce TRM cells in specific tissues or the administration of cytokines that promote TRM cell survival and activation. The challenges associated with targeting TRM cells, such as their limited accessibility in certain tissues and the risk of inducing autoimmunity, are also discussed.
Epigenetic regulation of TRM cells is another critical area of research explored in the article. Epigenetic modifications, such as DNA methylation and histone acetylation, play significant roles in the differentiation and function of TRM cells. These modifications help maintain the unique gene expression profiles required for TRM cell retention and activity within tissues. The article highlights ongoing research into how these epigenetic mechanisms can be modulated to enhance TRM cell-mediated cancer immunity. For instance, manipulating epigenetic marks to reinforce the TRM cell phenotype could improve their persistence and cytotoxic function in tumour tissues.
The relationship between TRM cells and the tumour microenvironment is another key theme in the article. The tumour microenvironment, which includes a variety of immune, stromal, and endothelial cells, can significantly impact TRM cell function. The article discusses how the immunosuppressive nature of the tumour microenvironment, characterised by the presence of regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and immunosuppressive cytokines such as IL-10 and TGF-β, can inhibit TRM cell activity and promote tumour immune evasion. Overcoming this suppression is a major challenge in enhancing TRM cell-mediated tumour control. Strategies to modulate the tumour microenvironment, such as the use of immune checkpoint inhibitors or therapies that deplete suppressive cell populations, are explored as potential approaches to enhance TRM cell function.
In conclusion, “Resident Memory T Cells and Cancer Immunity” provides a comprehensive analysis of the role of TRM cells in the immune response to cancer. The article emphasises the unique properties of TRM cells, their mechanisms of action, and their potential as therapeutic targets in cancer treatment. By discussing the importance of TRM cells in tumour immunosurveillance, control, and immunotherapy, the article offers valuable insights into how these cells can be harnessed to improve cancer outcomes. The exploration of epigenetic regulation and the challenges posed by the tumour microenvironment further underscores the complexity of TRM cell biology and the potential for innovative cancer therapies that target these cells.
Journal article: Damei, I., et al. 2023. Tumor-resident memory T cells as a biomarker of the response to cancer immunotherapy. Frontiers in Immunology.
Summary by Faith Oluwamakinde










