Macrophages are immune cells critical to the lungs’ defence and repair processes. These cells are present in healthy lungs and are rapidly recruited during injury or disease. While macrophages are key to initiating and resolving lung injuries, they are also implicated in pathways leading to lung scarring, or fibrosis—a process that can severely impair lung function. However, the mechanisms that determine whether macrophages contribute to repair or fibrosis remain unclear.
In a new study, researchers have gained insights into the role of lung macrophages in injury and repair, offering new perspectives on their involvement in fibrosis. In this study, they compared macrophages across multiple models of lung injury, reveals that macrophages traditionally labelled as “pro-fibrotic” are insufficient on their own to cause fibrosis. The findings open the door to research aimed at redirecting macrophage activity toward healthy lung repair.
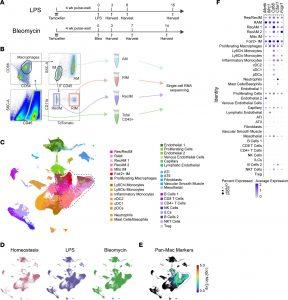
Figure 1: Macrophage isolation and scRNA-Seq following bleomycin and LPS-induced lung injury. (A) Timeline of interventions in experimental models. Tamoxifen was given to label resident interstitial macrophages followed by a 4-week wait period to allow clearance of labeled monocytes from the circulation. Mice were treated with i.t. LPS (20 μg) or bleomycin (1.5 U/kg) and were euthanized at the times indicated. Anti-CD45 antibody was instilled into the lungs immediately after euthanasia. (B) Four groups of cells were isolated from digested lung tissues using FACS. These included airspace macrophages (AMs), resident IMs (RIM), recruited IMs (RecIM), and general leukocytes (CD45+). AMs versus IMs were distinguished based on i.t. administered anti-CD45 labeling. Resident versus recruited IMs were distinguished by tdTomato expression. (C) Fully integrated UMAP including all sorted cells from day 0; LPS days 3, 6, and 15; and bleomycin days 3, 7, and 14. Clusters were manually annotated based on cluster-defining genes. Macrophage clusters are outlined in the dashed black line. (D) UMAP split to show homeostasis, LPS, and bleomycin samples. (E) Feature plot of the log2 minimum expression of mouse panmacrophage markers Fcgr1, C5ar1, CD68, Mrc1, and Mertk. (F) Average expression and percent of cells expressing panmacrophage marker genes in each cluster. Macrophage clusters in the dashed black box were used in subsequent analyses.
The study compared macrophage activity in two models of lung injury: one that typically results in healthy repair and another that leads to fibrosis. By analysing macrophage populations over time, the researchers identified a subset of macrophages recruited in all types of lung injuries. The study used genetic analyses to examine differences in the transcribed genes of these macrophages. Interestingly, macrophages expressing the Gpnmb gene—historically associated with fibrosis—were found in both injury models, suggesting that their role in fibrosis may have been overstated. This population of macrophages was also identified in a variety of lung diseases, including asthma and COVID-19, which exhibit a wide range of recovery outcomes.
Further investigation revealed that the lung microenvironment significantly influences macrophage behaviour. Subtle changes in protein expression and macrophage survival, driven by environmental factors, activated distinct pathways in the fibrotic injury model that steered macrophages toward dysfunctional repair. This finding highlights the importance of external signals in determining macrophage activity and the subsequent repair or scarring of lung tissue.
By identifying and manipulating macrophage pathways, scientists may develop therapies that rebuild lung tissue and restore function in patients with previously untreatable fibrotic diseases.
Journal article: King, E.M., et al, 2024. Gpnmb and Spp1 mark a conserved macrophage injury response masking fibrosis-specific programming in the lung, JCI Insight.
Summary by Stefan Botha










