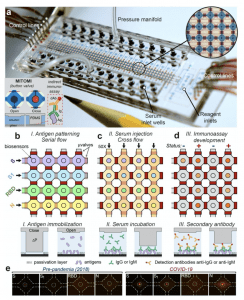
Figure 1. Microfluidic device for COVID-19 antibody detection. (a) Photograph of the device indicating its different components. The tip of a pencil is shown for reference. Closeup shows an array of microchambers (blue) surrounded by valves (red) and a MITOMI button valve (red circle) in the center. Bottom inset shows top-view photographs and a cross-sectional view of the actuation of the MITOMI button. An indirect immunoassay is carried out between on the surface of a glass substrate, dAb=detection antibody, Ig= serum antibodies, Ag= antigen. (b) Gross microfluidic assay schematic flow showing antigen immobilization of the four antigens, followed by (c) injection of the 50 samples and (d) immunoassay quantitation by the introduction of fluorescently labeled secondary antibodies. Solid or transparent red rectangles denote closing or opening of the microvalves, respectively. Bottom schematics show how MITOMI is used to perform an indirect immunoassay. S= Spike; S1 = subunit S1; RBD = Region Binding Domain; N = nucleocapsid. (e) Typical fluorescent micrographs of an array of biosensors for samples collected before the pandemic (left) and a COVID-19 confirmed case (right). (Source: Rodriguez-Moncayo et al. 2020).
Majority of (direct) SARS-CoV-2 diagnostics either detect nucleic acid or viral antigens in nasal or oral swabs, however, these diagnostics are most useful within the first three weeks of the onset of symptoms when most individuals have detectable viral burden. Antibody testing helps to confirm post-infections and are important for epidemiological studies. In this study, Garcia-Cordero’s lab have designed an indirect immunoassay microfluidic chip that can detect the presence of circulating IgM and IgG against main SARS-CoV-2 antigens in an automated high-throughput system.
The microdevice (Figure 1) detects circulating antibodies against of Spike (S), S1 subunit (S1), the Receptor Binding Domain (RBD), and Nucleocapsid (N) simultaneously and in an independent manner operated by microvalves which creates microchambers. The microdevices uses only 6 μL of serum to detect the presence of anti-SARS-Cov-2 antibodies from up to 50 samples (in one run) in less than 3 hours with a limit of detection comparable to an ELISA and a high sensitivity (90-95%) and specificity (88-94%). Authors report that “the accuracy of the assay increased to 100% when analyzing samples acquired between 20-30 days after symptom onset, consistent with other reports”. This is not surpising because its measuring antibody immunity which requires time to develop, especially for IgG.
Current authorized antibodies test is still expensive for some low- and middle-income countries. Thus, this kind of microdevices can be quickly applied to monitor the adaptative responses naturally or induced by the upcoming vaccines in with a very low cost due the reduced reagents volume in massive qualitative and semi-quantitative screenings.
Journal Article: Rodriguez-Moncayo et al. 2020. A high-throughput multiplexed microfluidic device for COVID-19 serology assays. Lab on a Chip
Article by Juan Carlos Balandran










