Stem cells are unique in their ability to develop into various types of specialized cells, which makes them invaluable for repairing tissues and generating new cells in the body. However, for these cells to thrive—particularly when transplanted into a patient—they need to evade the immune system’s watchful eye. If recognized as foreign, the immune system can mount an attack, leading to rejection and treatment failure.
A new study published has revealed the sophisticated strategies stem cells use to avoid detection and destruction by the immune system (Figure 1). This discovery sheds light on how certain stem cells survive in so-called “immune-privileged” zones of the body, offering new opportunities for advancing regenerative medicine and transplant therapies.
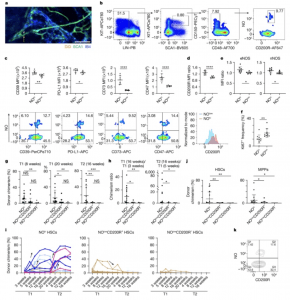
Figure 1: NO expression distinguishes distinctly potent, late-rising HSCs from less potent, exhausted HSCs. a, Histological image of allogeneic HSCs that persist in the skull BMs of non-conditioned immunocompetent recipients for 20 days after transplantation. DiD-labelled allogeneic HSCs (orange; SCA1+DiD+; indicated by the yellow arrow) persist at transitional regions from arterioles (narrow; green) into type-H vessels (wider; blue) at the endosteal surface. Blue, Alexa Fluor 488–IB4; green, Alexa Fluor 568–SCA1; red, DiD. Scale bar, 20 µm. b, The gating strategy used to identify NOhi HSCs from BM cells. c, CD39, PD-L1, CD73 and CD47 levels on NOhi HSCs. Top, median fluorescence intensities (MFIs). NOhi HSCs (NOhi) comprise HSCs for which the DAF-FM signal was in the top 15% levels. NOlow HSCs (NOlow) comprise HSCs in the bottom 85%. Bottom, representative flow cytometry plots of HSCs. d, CD200R levels on NOhi HSCs. Top, MFI ratios to the average MFIs of NOlow HSCs. Bottom, representative histograms. e, Levels of eNOS and nNOS in NOhi HSCs. y axis, ratio of MFI to the average MFIs of NOlow HSCs. f, Ki67+ frequencies. g–k, Competitive transplantation of NOhi, NOlowCD200R+ or NOlowCD200R− HSCs (CD45.1) (Extended Data Fig. 2a). g, CD45.1+ blood chimerism at 8 weeks after the first transplantation (T1), 20 weeks after the first transplantation and 16 weeks after the second transplantation (T2). h, Chimerism ratios at two different time points. Left, chimerism from 16 weeks after T1 to 3 weeks after T1. Right, chimerism at 16 weeks after T2 to 5 weeks after T1. i, Kinetics of donor chimerism. The colour of the trend lines corresponds to those of the reconstitution patterns in Extended Data Fig. 2d. The arrows denote increasing reconstitution trends in NOhi HSC recipients or exhausting trends in NOlowCD200R+ HSC recipients. j, CD45.1+ chimerism in HSCs and MPPs (LIN−SCA1+KIT+) at 20 weeks after the first transplantation. k, Representative flow cytometry plots of CD45.1+ HSCs in NOhi HSC recipients at 20 weeks after the first transplantation. Data are mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS, not significant (P > 0.05). See Supplementary Notes for sample sizes, experimental replicates and statistical analyses. See source data for individual P values.
Until now, the exact mechanisms by which stem cells escape immune detection were unclear. This new research highlights an elegant system that allows stem cells, particularly hematopoietic stem cells (HSCs), to remain hidden and protected.
The researchers focused on hematopoietic stem cells, which are responsible for generating the body’s entire supply of blood cells. These cells are already used in treatments for leukemia and inherited blood disorders. Yet, one major obstacle has been preventing the immune system from rejecting them.
In this study, NOhi HSCs, produce high levels of nitric oxide (NO), a molecule that appears to play a crucial role in modulating the immune system. Nitric oxide isn’t just a signalling molecule—it also helps these HSCs suppress immune activity around them. The researchers found that NOhi HSCs express high levels of CD200R, a receptor known to dampen immune responses and promote immune tolerance. This combination allows NOhi HSCs to avoid immune attack and ensures their survival and proliferation.
These immune-privileged stem cells aren’t just floating freely in the bloodstream; they cluster near specialized capillaries with distinctive hairpin curves. These curved blood vessels alter shear stress, the force exerted by blood flow along vessel walls. The researchers found that increased shear stress helps regulate nitric oxide production in these stem cells.
This mechanical cue, combined with NO production, creates a protective microenvironment that allows NOhi HSCs to thrive. Traditionally, blood vessels are viewed as passive conduits for blood. However, this study highlights a new role for blood vessels as active regulators of stem cell survival and immune privilege. Essentially, these blood vessels serve as gatekeepers, helping maintain a balance between immune tolerance and stem cell function.
Key Takeaways from the study:
- Researchers have discovered how NOhi hematopoietic stem cells (HSCs) evade immune attacks by producing nitric oxide and expressing the immune-modulating receptor CD200R.
- These stem cells survive in regions near specialized curved blood vessels, where shear stress regulates their behavior and nitric oxide production.
- The findings redefine the role of blood vessels as active regulators of immune privilege, not just conduits for blood.
- Insights from this study could pave the way for new regenerative therapies, better transplant outcomes, and novel cancer treatments.
This study not only solves a longstanding puzzle in stem cell biology but also offers a promising blueprint for developing immune-smart therapies in the future.
Journal article: Furuhashi, K., et al., 2025. Bone marrow niches orchestrate stem-cell hierarchy and immune tolerance. Nature.
Summary by Stefan Botha










