As we age, our immune systems undergo changes that lead to chronic inflammation, even in the absence of pathogens. This chronic, low-grade inflammation damages tissues and makes older individuals more vulnerable to infections and a range of diseases such as cancer, type 2 diabetes, and heart disease. In a recent study, scientists have identified a key signalling molecule that, when lacking in certain immune cells, can induce age-related diseases in young mice (Figure 1). This finding has the potential to contribute to the development of novel treatments for age-related conditions.
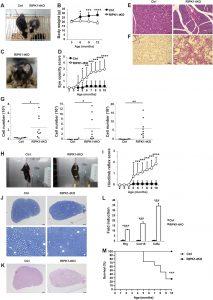
Figure 1: RIPK1-tKO mice develop inflammatory disease. (A) Representative photograph showing the appearance of control and 10-month-old RIPK1-tKO mice (right). (B) Body weight changes over time in control (n = 6) and RIPK1-tKO female mice (n = 8). (C) Representative photograph of a 10-month-old RIPK1-tKO mouse that has developed opacity of the eyes. (D) Eye opacity score of control (n = 10) and RIPK1-tKO (n = 13) mice. Representative hematoxylin and eosin (H&E)–stained sections of salivary glands (E) and lung (F). Scale bars, 100 μm (E) and 50 μm (F). (G) Quantification of total cells (left), eosinophils (middle), and neutrophils (right) in the BAL fluid from 7- to 10-month-old control and RIPK1-tKO mice (n = 9 per group). (H) Representative photograph of 8-month-old control and RIPK1-tKO mice suspended by the tail. RIPK1-tKO mice clench their hindlimbs to the body. (I) Hindlimb reflex score of control and RIPK1-tKO mice (n = 13 per group). (J) Toluidine blue–stained semithin transverse sections of the sciatic nerve from 8-month-old control and RIPK1-tKO mice. Scale bars, 50 μm (top) and 20 μm (bottom). (K) H&E-stained semithin transverse sections of the sciatic nerve from 8-month-old control and RIPK1-tKO mice. Scale bars, 50 μm. (L) Quantitative polymerase chain reaction (qPCR) analysis for the expression of Ifng, Cxcl10, and Cd3e in sciatic nerve from 10-month-old control and RIPK1-tKO mice. (M) Survival of control (n = 8) and RIPK1-tKO (n = 11) mice. Error bars represent SEM. Data are representative of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.001 using Student’s t test.
The phenomenon known as “inflammaging” involves an overactive state of T cells, which are responsible for recognizing and responding to specific pathogens. This overdrive state of T cells is referred to as senescence.
The signaling molecule in question is called receptor-interacting protein kinase 1 (RIPK1), which controls cell death through different pathways depending on the compounds it interacts with. Previous studies have shown that individuals with a deficiency in RIPK1 are more prone to inflammatory disorders.
The researchers discovered how premature inflammaging occurs. In the absence of RIPK1, two compounds, caspase-8 and RIPK3, excessively activate a cell-growth regulator called mTORC1. This activation, in turn, leads to T cell senescence.
By understanding the mechanisms underlying inflammaging and the role of RIPK1, researchers can potentially identify targets for therapeutic intervention.
Journal article: Takayuki Imanishi, T., et al, 2023. RIPK1 blocks T cell senescence mediated by RIPK3 and caspase-8. Science Advances.
Summary by Stefan Botha










