Introduction
The article titled “The Neuroimmunology of Social-Stress-Induced Sensitization” (Figure 1) delves into the intricate relationship between chronic social stress, inflammation, and mood disorders, particularly focusing on mechanisms of stress sensitization observed in conditions such as Post-Traumatic Stress Disorder (PTSD). Authored by Rebecca G. Biltz, Caroline M. Sawicki, John F. Sheridan, and Jonathan P. Godbout, the study emphasises the physiological, immunological, and behavioural responses that contribute to prolonged anxiety, social withdrawal, and cognitive impairment, especially in the context of repeated social defeat (RSD) in rodents.
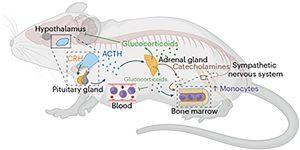
Figure 1: Social defeat stress activates the hypothalamic pituitary adrenal axis and sympathetic nervous system to promote the release of monocytes into circulation. Social defeat stress increases the release of corticotrophhin-releasing hormone (CRH) in the hypothalamus. The release of CRH prompts the pituitary gland to release adrenocorticotropic hormone (ACTH) into circulation. In turn, the adrenal glands respond and release both glucocorticoids and catecholamines. Glucocorticoids feed back to the hypothalamus to stop the production of CRH. Parallel to this, social defeat activates the SNS. SNS activation results in the release of catecholamines (for example, norepinephrine) that act directly on primary and secondary lymphoid tissues (for example, bone marrow). Both glucocorticoids and catecholamines converge to increase the production, maturation, and release of monocytes into circulation. Created with BioRender.com.
The Impact of Chronic Stress on Neuroimmunology
Chronic stress is known to have significant psychological and physiological effects, often leading to increased inflammation, which in turn exacerbates mental health issues like anxiety and depression. The article highlights how chronic stress can lead to stress sensitization, where individuals become more vulnerable to subsequent stressors, resulting in long-term complications. This is particularly relevant in PTSD, where stress sensitization alters neuronal circuitry and mood, though the exact mechanisms remain unclear.
Rodent Models of Social Defeat
Rodent models, particularly those involving repeated social defeat (RSD), have been instrumental in understanding the neuroimmunology mechanisms underlying stress sensitization. These models mimic the chronic social stress experienced by humans, leading to increased anxiety, social withdrawal, and cognitive deficits in the rodents. RSD, a severe form of social stress, causes a convergence of neuronal, central inflammatory (microglial), and peripheral immune (monocyte) pathways, contributing to prolonged anxiety and stress sensitization.
Immune System’s Role in Stress Sensitization
One of the key findings discussed in the article is the role of the immune system in stress sensitization. Chronic stress leads to alterations in the communication between the brain and the immune system, with significant changes in both peripheral and central immune responses. For instance, chronic stress can increase the production of pro-inflammatory monocytes, which are associated with anxiety and depressive symptoms. These monocytes are more resistant to glucocorticoid regulation, resulting in increased production of pro-inflammatory cytokines.
Microglia and Neuroinflammation
Microglia, the central nervous system’s primary immune cells, also play a crucial role in stress sensitization. In response to chronic stress, microglia become activated and adopt a pro-inflammatory profile, which has been linked to various psychiatric disorders, including depression, anxiety, and PTSD. The article discusses how structural alterations in microglia and increased inflammatory profiles in brain regions associated with cognition and memory, such as the hippocampus and prefrontal cortex, contribute to the severity of these disorders.
Social Defeat Stress and Anxiety Behaviours
The article further explores the behavioural consequences of social defeat stress in rodents, which closely parallel the mood disorders observed in humans. RSD leads to anxiety behaviours such as social avoidance and neophobia, which are mediated by the recruitment of monocytes to the brain by activated microglia. This process results in an exaggerated inflammatory response, even to subthreshold stressors, weeks after the initial stress exposure, highlighting the long-term impact of stress sensitization.
Mechanisms of Stress Sensitization
The mechanisms underlying stress sensitization involve a complex interplay between the central nervous system and the immune system. The activation of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis during chronic stress leads to the release of catecholamines and glucocorticoids, which directly affect immune cell production and release. However, chronic stress can dysregulated this response, resulting in glucocorticoid resistance and increased inflammation.
The article also discusses how social defeat stress leads to the production and release of inflammatory monocytes, which traffic to the brain and contribute to the development of anxiety-like behaviours. These monocytes, characterised by a pro-inflammatory profile, are recruited to the brain by microglia through the expression of chemokines and cell adhesion molecules. The subsequent accumulation of these monocytes in the brain, particularly in regions associated with fear and threat appraisal, is a critical factor in the persistence of anxiety and the development of stress sensitization.
Clinical Implications and Future Directions
The findings from this study have significant clinical implications, particularly in understanding and treating conditions like PTSD. By delineating the pathways involved in stress sensitization, particularly the role of the immune system, the study provides insights into potential therapeutic targets for preventing or mitigating the long-term effects of chronic stress.
In conclusion, the article “The Neuroimmunology of Social-Stress-Induced Sensitization” provides a comprehensive overview of the mechanisms by which chronic social stress leads to stress sensitization and its associated neuropsychiatric complications. Using rodent models, the study highlights the critical role of the immune system, particularly monocytes and microglia, in mediating the long-term effects of stress. These findings underscore the importance of further research into the neuroimmune interactions that underlie stress-related disorders, with the goal of developing more effective treatments for conditions like PTSD.
Journal article: Montagud-Romero., S., et al., 2021. Unravelling the Neuroinflammatory Mechanisms Underlying the Effects of Social Defeat Stress on Use of Drugs of Abuse . Neuroscience of Social Stress.
Summary by Faith Oluwamakinde
References:
- Biltz, R. G., Sawicki, C. M., Sheridan, J. F., & Godbout, J. P. (2022). The neuroimmunology of social-stress-induced sensitization. Nature Immunology, 23(11), 1527–1535. https://doi.org/10.1038/s41590-022-01248-6
- McEwen, B. S. (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology, 583(2-3), 174-185. https://doi.org/10.1016/j.ejphar.2007.11.071
- Dantzer, R., & Kelley, K. W. (2007). Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity, 21(2), 153-160. https://doi.org/10.1016/j.bbi.2006.09.006
- Wohleb, E. S., Hanke, M. L., Corona, A. W., Powell, N. D., Stiner, L. M., Bailey, M. T., … & Godbout, J. P. (2011). β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. Journal of Neuroscience, 31(17), 6277-6288. https://doi.org/10.1523/JNEUROSCI.0450-11.2011
- Fanselow, M. S., & Dong, H. W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65(1), 7-19. https://doi.org/10.1016/j.neuron.2009.11.031










