With the initiation of ART during pregnancy, there have been significant strides made when it comes to combating mother-to-child transmission (MTCT) of HIV. Due to the shortcomings that the ART strategy is still prone to, we see that there are still infants that do end up getting newly infected with HIV. To aid current efforts to reduce MTCT, the authors propose the concept of maternal vaccination to help boost the effectiveness of ART.
As non-human primates have been a good avenue that researchers have used to understand more about MTCT of HIV, Nelson et al decided to use a non-human primate model (figure 1) to look at vaccination with HIV Env in the context of maternal HIV infection and recent ART initiation during the period of gestation. They assessed how an HIV Env vaccine regimen could enhance protective maternal Env-specific antibody responses in SHIV-infected, ART suppressed, non-pregnant female rhesus macaques (RMs).
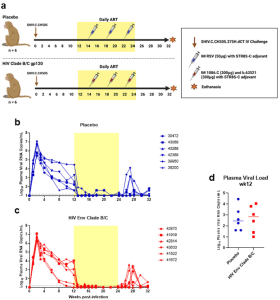
Figure 1: Infection, treatment, and immunization schedule for study of vaccine-elicited responses in SHIV-infected, ART-treated female monkeys to reduce MTCT. a Study design schematic. Twelve female RMs were infected with SHIV.C.CH505 (brown arrow), started on a daily ART regimen at 12 wpi (yellow box). Six RMs received either a placebo vaccine (blue syringes) or a HIV Env combined clade B/C gp120 vaccine (red syringes). ART was discontinued at 24 wpi, and RMs were monitored for an additional 8 weeks. Plasma viral RNA kinetics through 32 wpi for placebo vaccinated (b) and HIV Env Clade B/C gp120 (c) vaccinated RMs. Plasma vRNA load at 12 wpi, prior to ART start, in placebo (blue) and HIV Env (red) vaccinated RMs (d). Medians are represented by the horizontal lines.
Nelson et al were able to show that Env vaccination in SHIV-infected, ART-suppressed RMs did enhance IgG responses against the vaccine immunogens b.63521 and 1086.c, as well as against the challenge virus Env, SHIV.C.CH505 when compared to the placebo group (figure 2).
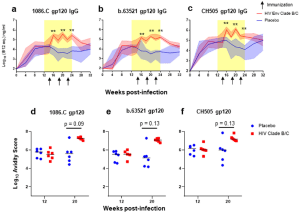
Figure 2: Plasma gp120-specifc IgG antibody binding kinetics, avidity, and breadth. Plasma gp120 IgG binding responses to vaccine antigens 1086.C (a) and b.63521 (b), and challenge virus antigen, CH505 (c) in placebo (blue) and Env vaccinated (red) macaques. Bold lines represent the median, and the range is depicted by the shaded area; black arrows indicate time points at which immunizations were administered (14, 18, and 22 wpi). Antibody avidity was assessed against vaccine and challenge antigens at week 12, pre-ART/immunization, and at week 20 when IgG gp120 binding peaked in the Env vaccinated group (d–f). Dot plots represent avidity score, each dot represents one animal, and medians are represented by horizontal lines (d–f). Statistical analysis was performed using Wilcoxon rank-sum tests with exact p-values to compare gp120-specifc IgG binding and avidity responses between vaccinated and placebo RMs, followed by FDR adjustments for multiple comparisons. **unadjusted p < 0.01. FDR adjusted p-values are reported in the dot plots.
The authors note though, that in spite the enhancement in antibody responses that were specific for Env, HIV Env vaccination did not seem to stop or postpone viral rebound time. Nelson et al suggest that the initiation of ART at a later time point, that is 12 weeks post infection, could possibly account for these results.
There were no effective neutralization responses that came up from the Env vaccine and so the authors suggest that other approaches may need to be given a try when it comes to stimulating the B-cell responses required in ART-suppressed, HIV-infected pregnant women. A look into the non-neutralizing functions, however, did show that there was a boost in (antibody-dependent cellular cytotoxicity) ADCC-mediated antibodies in RMs that were vaccinated. More research work that comes up in relation to ADCC-mediated antibodies would help better determine whether these responses could also contribute to reduced MTCT risk.
Overall, this is what the authors had to say; “our results suggest that a universal maternal HIV vaccine regimen can be developed to boost antibodies that target the maternal autologous virus pool, and elicit previously identified humoral immune correlates of reduced MTCT risk in humans”. Nelson et al also adds that the next and much needed steps in this line of research require developing pregnancy models to find out whether vaccination can generate protective immune responses in pregnant women similar to what was seen here, since it is clear that pregnancy can alter the maternal immune environment.
Journal article: Nelson, A.N., et al., 2022. Leveraging antigenic seniority for maternal vaccination to prevent mother-to-child transmission of HIV-1. NPJ Vaccines.
Summary by Vanessa Muwanga










