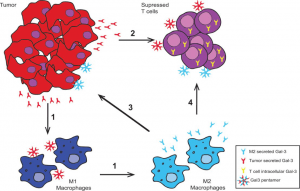
Impact of Gal-3 within the TME. Gal-3 is secreted by the tumor cells as monomers, which can form pentamers and bind substrates. The arrows indicate the influence of extracellular Gal-3 on various cell subsets. Gal-3 secreted by tumor cells: 1) polarizes M1 macrophages to M2 macrophages and 2) suppresses CD4 and/or CD8 T cells. Gal-3 secreted by M2 macrophages: 3) binds tumor cells to promote tumor progression/metastasis and 4) suppresses T cells. (Source: Farhad et al., 2020)
Galectin-3 (gal-3), member of the galectin family, is a potential biomarker and therapeutic target in numerous types of cancer. Lectins family members in animals have been implicated in a plethora of cellular processes like: adhesion, migration, survival, death and differentiation. As with the other family members, gal-3 is ubiquitously expressed and is specifically binds β-galactose-containing-glucoconjugates through a specific region named carbohydrate carbohydrate-recognition-binding domain (CRDs). It is well documented that gal-3, produced by tumor cells or immune cells such as M2 macrophages, could have a pro-tumoral activity and participate to tumoral escape from immune responses. In this review, the authors underlying potential immunosuppressive effect of gal-3 through his modulation of lymphocytes and macrophages activity, specifically in the prostate and lung cancer.
Depending on its location, extracellular or intracellular, gal-3 can show pro-or anti apoptotic effect on lymphocytes. Extracellular gal-3 induce thymocytes and T lymphocytes apoptosis due to the stimulation of CD45 or CD71, while intracellular gal-3 inhibit this death process by binding to bcl-2 protein. Gal-3 has also shown an ability to down regulate TCR expression and INF-γ secretion of both CD4 and CD8 T cells. On other hand, Gal-3 can turn classical M1 macrophages into immunosuppressive M2 macrophages.
Despite the known immunosuppressive effect of gal-3 in the tumor microenvironment, its expression is negatively correlated with prostate cancer progression while in non-small Lung cell cancer, high gal-3 expression is associated with metastasis.
The overwhelming evidence of Gal-3 involvement in boosting tumor growth, metastasis, and immune suppression has made Gal-3 an exciting target for cancer immunotherapy. Gal-3 inhibitors, such as GRMD-02 and GCS-100, used alone or in combination with existing immunotherapies have shown promising results in preclinical and clinical trials.
Journal Article: Farhad et al., 2018. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology
Summary by Doudou Georges Massar Niang










