Neutrophils have been shown to control T. gondii infection, which is chronically in approximately one-third of the human population. T. gondii infection is established in the small intestine from consumption of contaminated food or water and after entering of the bloodstream, T. gondii disseminates through different organs, also with the help of infected immune cells to reach immunoprivileged sites (for more on neutrophils read – Neutrophils on Immunopaedia).
Mice are a natural intermediate host for T. gondii and therefore became a popular model to study immunological host defense. However, mice and humans have not always the same immunological mechanisms and studies of human neutrophils in T. gondii infection are scarce. Miranda et al. now determined the activation status of human neutrophils from patients with acute and chronic infections of T. gondii by flow cytometry. Additionally, they investigated the induction of neutrophil extracellular trap (NET) formation, the production of cytokines/chemokines and the recruitment of adaptive effector cells upon T. gondii stimulation (Figure 1).
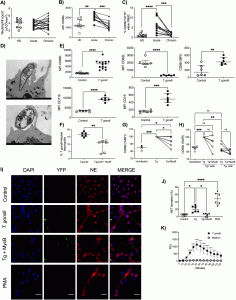
Figure 1: T. gondii induces neutrophil activation and NET formation. (A to C) Neutrophil absolute counts in whole blood (A), scatter dot plots of the activation marker CD66b (B), and frequency of CD66b+ CD16+ cells (C) in PBMCs from HDs (n = 9 to 11) and acutely (n = 11 to 21) and chronically (n = 11 to 21) T. gondii-infected patients. Each pair connected by a line represents a single individual. (D) Representative transmission electron microscopy images from a T. gondii active infection and a T. gondii opsonized infection of neutrophils from HDs. Bars, 500 nm. (E) Expression of CD66b, CD11b, CD15, CD62L, and CD88 by neutrophils from HDs in medium alone (n = 6 to 13) or stimulated with T. gondii tachyzoites (n = 6 to 14). (F) Relative expression of CD66b by neutrophils from HDs in medium alone or stimulated with T. gondii tachyzoites without (Tg) or with (Tg+MycB) mycalolide B pretreatment (n = 6). (G) Percentage of neutrophils infected for 4 h with T. gondii or T. gondii pretreated with mycalolide B (3 μM, 30 min) (n = 6). (H) Relative CD66b expression in the different subpopulations (Tg positive and negative) from panel G. (I) Representative immunofluorescence microscopy and (J) scatter dot plots (n = 6) of neutrophils from HDs in medium alone (Control), exposed to T. gondii RH expressing YFP with or without pretreatment with mycalolide B (3 μM, 30 min), or stimulated with 25 nM Phorbol 12-myristate 13-acetate (PMA) for 3 h. Cells were stained with Hoechst and anti-human neutrophil elastase (NE). Bars, 25 μm. (K) Neutrophil extracellular ROS production was measured by chemiluminescence (relative light units [RLU]) for 60 min in neutrophils from HDs that were unstimulated (n = 5) or stimulated with T. gondii (n = 5). The differences are relative to the control. The T. gondii-neutrophil ratio was 3:1. Data are medians and interquartile ranges. *, 0.05 > P > 0.01; **, 0.01 > P > 0.001; ***, 0.001 > P > 0.0001; ****, P < 0.0001; ns, not significant (Miranda, et al., 2021).
Neutrophils from acute infected patients were significantly activated as shown by the upregulation of CD66b and the presence of CD66+CD16+ cells in the PBMC fraction. Transmission electron microscopy further revealed that tachyzoites actively infect neutrophils probably through phagocytosis. Moreover, the frequency of NET formation and ROS production was increased in neutrophils from healthy donors exposed to T. gondii. These NETs further amplified the innate immune response by promoting the migration and activation of neutrophils as shown using NET supernatants. Importantly, the T. gondii induced NETs not only activated more neutrophils but amplified also the adaptive immune response by attracting T cells and inducing the production of inflammatory cytokines by PBMCs.
Journal Article: Miranda, et al., 2021. Toxoplasma gondii-Induced Neutrophil Extracellular Traps Amplify the Innate and Adaptive Response. American Society for Microbiology.
Summary by Dr. Jasmin Knopf (Friedrich-Alexander-University of Erlangen-Nürnberg, Rheumatology and Immunology Clinic)










