Nguyen et al. published a review article describing the use of immunoglobulins as a treatment for COVID-19. As COVID-19 cases rise at a rapid rate, there is great need to explore pre-existing treatments while novel treatments and vaccines are being developed. Such pre-existing treatments include Intravenous Immunoglobulins (IVIG) and Hyperimmune globulins.
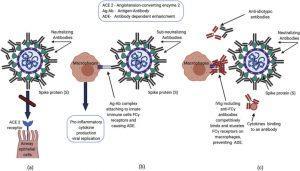
Proposed mechanisms of neutralizing antibodies and IVIG in COVID-19 infection.(a) Neutralizing antibodies prevent SARS-CoV2 spike protein from attaching to the ACE2 receptor, inhibiting viral entry into the cell. (b) Immune complexes consisting of viral antigens and anti-viral sub-neutralizing antibodies can activate Fcγ receptors on innate immune cells (e.g. macrophages) in the lung, triggering an exaggerated inflammatory response leading to acute lung injury via antibody dependent enhancement (ADE). Additionally, antibody-bound virus can be internalized through Fcγ receptors, enhancing viral replication. (c) Proposed mechanisms whereby IVIG exerts anti-inflammatory action include saturation of Fcγ receptor binding, anti-idiotypic binding to anti-viral antibodies, and binding of proinflammatory cytokines. (Source: Nguyen et al., 2020)
Antiviral antibodies produced during SARS-CoV2 infection block the virus from binding the ACE2 receptor, thus preventing viral entry into the host cell. However, production of viral antibodies have been shown to aggravate (other) disease(s) through Antibody Dependent Enhancement (ADE). This occurs when immune complexes consisting of viral antigens and anti-viral sub-neutralizing antibodies bind onto the FCγ receptors of the M2 macrophages in the lungs. This results in exaggerated inflammatory responses characterized by the production pro-inflammatory cytokines and recruitment of inflammatory cells leading to acute lung injury and enhanced viral replication. ADE poses a potential challenge in the development of COVID-19 vaccine, however this pathological phenotype is yet to be described in COVID-19 pathology (Coish & MacNeil, 2020; Arvin et al., 2020)
In theory Immunoglobulins present could prevent ADE by saturating the FCγ receptors, binding to anti-viral antibodies or pro-inflammatory cytokines. Several studies have shown improvement in SARS and H1N1 patients after receiving immunoglobulins, albeit, these studies had confounding variables as a majority of patients were also treated with antivirals and corticosteroids. It was observed that patients who received hyperimmune globulin had significantly reduced viral loads and increased survival compared to the group receiving IVIG, hence indicating the superiority of Hyperimmune globulins over IVIG. These studies lacked case matched controls, though, performing randomised control studies including a placebo arm is a challenge during the COVID-19 pandemic.
In conclusion, the authors suggest “plasma studying the therapeutic potential of sera from convalescent patients who experience milder infection, without ADE, and the antibody titres be verified to ensure adequacy.” Nonetheless, without controlled studies, the therapeutic benefits of immunoglobulins remains inconclusive.
Journal Article: Nguyen, A. A. et al. (2020). Immunoglobulins in the treatment of COVID-19 infection: Proceed with Caution! Clinical Immunology.
Summary by Vicky Gent
Also Read










