As of today, January 31, 2021, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 375 million individuals globally and lead to more than 5,5 million deaths. Today the virus has been known to cause a variety of symptoms resembling flu and in certain circumstances may progress to death.
To date, the function of interferon-λ (IFN-λ) in SARS-CoV-2 infection and subsequent disease pathology remains unknown. Patients with the disease, COVID-19, have higher amounts of pro-inflammatory cytokines and chemokines in their circulation and they have lower levels of type I and III IFN. This is suggestive of virus-induced antagonism or skewing of antiviral responses.
It has been reported that elevated levels of IFN-λ in serum and were linked to a reduction in viral infection within the respiratory tract and efficient viral clearance in one human trial. In addition, a higher ratio of IFN-λ to type I IFN was linked to a better survival outcome.
In a recent paper, Chong, et al., investigated the effect of IFN-λ on SARS-CoV-2 infection in mice. In this present study, the authors found that IFN-λ conferred protection against the SARS-CoV-2 B.1.351 (Beta variant) and the newer SARS-CoV-2 B.1.1.529 (Omicron variant). The authors also decided to test exogenous intranasally administered IFN-λ2 and its role in protecting mice against SARS-CoV-2 infection for which they had similar findings (Figure 1 and 2). IFN-λ2 treatment resulted in decreased viral RNA levels in the nasal turbinates, nasal washes, and lungs, but not in the brain. Testing therapeutic efficacy of IFN-λ2, showed that in the nasal turbinates, lungs, and brains of IFN-λ2 treated mice, viral RNA levels were lower, as were infectious virus titers in the nasal turbinates and lungs.
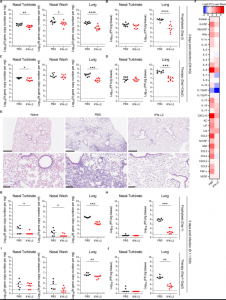
Figure 1: Nasally-delivered IFN-λ2 treatment protects K18-hACE2 mice against SARS-CoV-2 infection. Eight-week-old female K18-hACE2 mice were inoculated by intranasal route with 103 FFU of WA1/2020 D614G. At D-2 (A-B) or D+1 and D+2 (C-D), mice were given a single 2 μg dose of murine IFN-λ2 or PBS by the intranasal route. (A and C) Viral RNA levels were measured at 3 dpi. (B and D) Infectious virus was measured at 3 dpi (A-B: n = 9 per group, 2 experiments; C-D: n = 8 per group, 2 experiments). (E) Hematoxylin and eosin staining of lung sections from animals treated with 2 μg doses of murine IFN-λ2 or PBS by intranasal route at -16 h and +8 h relative to inoculation with WA1/2020 D614G and harvested at 7 dpi. Low (top, scale bars, 500 μm) and high (bottom, scale bars, 100 μm) power images are shown. Representative images from n = 5 per group. (F) Eight-week-old female K18-hACE2 mice were treated with 2 μg of murine IFN-λ2 or PBS at available under aCC-BY-NC-ND 4.0 International license. (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made bioRxiv preprint doi: https://doi.org/10.1101/2022.01.21.477296; this version posted January 24, 2022. The copyright holder for this preprint 23 -16 h and challenged with 103 FFU of WA1/2020 D614G. Heat-maps of cytokine levels in lung homogenates at 3 dpi. Fold-change was calculated relative to mock-infected mice, and log2 values are plotted (2 experiments, n = 7 per group except naïve, n = 4). (G-J) Five-month-old female K18-hACE2 mice were inoculated with 103 FFU of B.1.1529. At D-1 (G-H) or D+1 and D+2 (I-J), mice were given 2 μg of murine IFN-λ2 or PBS by the intranasal route. Viral RNA (G and I) and infectious (H and J) virus levels were measured at 3 dpi (G-H: n = 7-8 per group, 2 experiments; I-J: n = 6-7 per group, 2 experiments). Bars (A-D and G-J) indicate median values. Data were analyzed by Mann-Whitney tests (A-D and G-J) (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001) (Chong, et al., 2022).
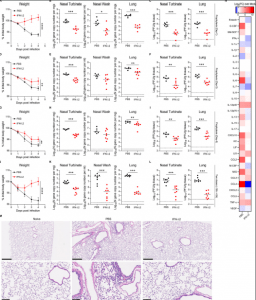
Figure 2: IFN-λ2 treatment protects 129S2 mice against SARS-CoV-2 infection. (A-I) Six-week-old female 129S2 mice were inoculated by intranasal route with 105 FFU of B.1.351. At D-1 (A-C), D-3 (D-F) or D-5 (G-I), mice were given a single 2 μg dose of murine IFN-λ2 or PBS by intranasal route. (A, D, G) Weight change. (B, E, H) Viral RNA levels at 4 dpi. (C, F, I) Infectious virus levels at 4 dpi (A-C: n = 7 per group, 2 experiments; D-F: n = 6-8 per group, 2 experiments; G-I: n = 6-8 per group, 2 experiments). (J-L) Six-week-old female 129S2 mice were inoculated by intranasal route with 105 FFU of B.1.351. At -16 h and +8 h, mice were administered 2 μg of murine IFN-λ2 or PBS by intranasal route. (J) Weight change. (K) Viral RNA levels at 4 dpi. (L) Infectious virus levels at 4 dpi in (n = 8 per group, 2 experiments). (M) Hematoxylin and eosin staining of lung sections at 4 dpi from animals treated in (J-L). Low (top, scale bars, 500 μm) and high (bottom, scale bars, 100 μm) power images are shown (representative of n = 5 per group). (N) Heat-maps of cytokine levels in lung homogenates at 4 dpi from animals treated in (J-L). available under aCC-BY-NC-ND 4.0 International license. (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made bioRxiv preprint doi: https://doi.org/10.1101/2022.01.21.477296; this version posted January 24, 2022. The copyright holder for this preprint 2 Fold-change was calculated compared to mock infected mice, and log2 values were plotted (n = 8 per group except naïve n = 4, 2 experiments). Bars (B-C, E-F, H-I and K-L) indicate median values. Data were analyzed by Mann-Whitney tests (B-C, E-F, H-I and K-L) or t tests of the area under the curve (A, D, G and J) (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001) (Chong, et al., 2022).
In their own words:
“In summary, we present evidence that nasal administration of IFN-λ confers pre- and post-exposure protection against infection by several SARS-CoV-2 strains including key variants of concern without causing extensive inflammation. In the lung, IFN-λ is induced in a MAVS and MyD88-dependent manner primarily in ECs that are likely infected, and acts upon radio-resistant cells to control infection. Additional treatment studies are warranted to evaluate further the potential of IFN-λ as a broadly-acting antiviral agent against SARS-CoV-2 and its emerging variants.”
NB to note: bioRxiv is a preprint server which publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, or guide clinical practice or treated as established information.
Journal article: Chong, Z, et al., 2022. Nasally-delivered interferon-λ protects mice against upper and lower respiratory tract infection of SARS-CoV-2 variants including Omicron. bioRxiv.
Summary by Stefan Botha










