Melanoma is the deadliest form of skin cancer with increasing incidence globally. Checkpoint inhibitors (CI) are among the novel systemic therapies demonstrated to have significantly improved outcomes of melanoma patients. Indeed, James P. Allison and Tasuku Honjo were awarded the Nobel Prize in Physiology or Medicine in 2018 owing to their contributions in the development of CIs – a therapeutic approach that mainly targets exhausted T lymphocytes located within the tumor tissue-. Monoclonal antibodies constitute the currently available CIs, playing a key role in blocking the regulatory functions of either the PD1/PD-L1 pathway or CTLA-4 in tumor melanomas.
However, current CIs based-therapies have shown limitations including a non-negligible proportion of patients irresponsive to treatment. Hitherto, this is likely to be a consequence of other leukocyte activities within the tumor microenvironment, precisely tumor associated macrophages (TAMs). TAMs are quantitatively the major immune cell population inside the tumor in many cancer types including melanoma. They mainly carry a pro-tumor activity and could promote CI therapy failure further making them interesting targets even out of a CI therapy context. In a review entitled “Influencing tumor-associated macrophages in malignant melanoma with monoclonal antibodies”, Rebecca Adams et al. described the involvement of tumor-associated macrophages (TAM) in failure of CI therapy and potential strategies to target those cells thereby improving CI efficacy.
In CI therapy, the most deleterious TAMs share some phenotypic and functional patterns with the alternatively activated macrophages that carry scavenger receptors and secrete IL-4, IL-13, VEGF and metalloproteinase. However, TAMs comprise highly plastic, heterogeneous and complex population for which the functions rely on the tumor microenvironment and their metabolic activity. They can support melanoma growth and dampen immunogenic killing of cancer. Furthermore, they have reduced phagocytosis and antigen presentation abilities (Figure 1).
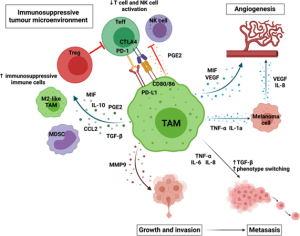
Figure 1: TAMs can promote melanoma growth and progression by creating an immunosuppressive immune environment by recruiting and maintaining immunosuppressive cells, such as Tregs, M2-like TAMs and MDSCs and reducing effector cells activation; promoting angiogenesis directly by secreting VEGF and MIF and indirectly through TNF-α and IL-1 α promoting angiogenesis by melanoma cells; enabling invasion by secreting metalloproteinases; and enabling metastasis by promoting the secretion of factors which increase phenotype switching (TGF-β), increase motility (IL-8), and increase invasion (IL-6). Created with BioRender.com.
The Fab region of monoclonal antibody (mAb) used in CI therapy binds to checkpoint molecules in order to neutralize their inhibitory effect on T cells. Besides, the Fc region is able to bind Fc receptors (FcRs) expressed by a wide range of leukocytes including TAMs. Rebecca Adams et al. reported that such mAbs bind both to checkpoint molecules expressed by melanoma cells or TAMs and Lymphocytes, and also to FcRs on TAM surfaces. The binding to checkpoint molecules and FcRs on TAMs surfaces usually leads to therapeutic failure. The mechanisms underlying that involve: (i) the competition between checkpoint molecules on melanoma cells and on TAMs for binding to mAbs; (ii) the killing by TAMs through Fc-mediated functions of antitumor lymphocytes expressing checkpoint molecules; (iii) secretion by TAMs of immunosuppressive and pro-angiogenesis molecules; (iv) regulatory T cells recruitment.
Due to their pro-tumor activity both in and out of the CI context, different strategies are under development and trial for targeting TAMs including: (i) inhibition of TAMs recruitment, (ii) suppression of pro-tumor functions of TAMs; (iii) promotion of antitumor function of macrophages; (iv) depletion of pro-tumor macrophage subsets.
In summary, the review by Rebecca Adams et al. demonstrated that lymphocytes are not the only leukocytes targetable within melanoma tumor tissues. In additionTAMs targeted therapy failed in providing sufficient efficacy to be approved as an alternative of the CI therapy. One explanation could be the complexity of tumor growth and metastasis mechanisms, and the redundancy that exist between pro-tumor processes in tumor microenvironment. Thus combining therapeutic strategies that target lymphocytes and other leukocytes or components of tumor microenvironment should be considered. This could become an interesting approach for both melanoma and other cancers.
Journal article: Adams, R., et al., 2022. Influencing tumor-associated macrophages in malignant melanoma with monoclonal antibodies. OncoImmunology.
Summary by Doudou Georges Massar Niang










