In a recent paper, researchers described the development of a novel oncolytic virus (OV) called OV-mOX40L for the treatment of pancreatic cancer (Figure 1). The study aimed to combine the direct oncolytic effect of OV with the immune-stimulating activity mediated by OX40L, a protein that activates T cells. The researchers conducted experiments using a syngeneic mouse model of pancreatic cancer (KPC mice) to evaluate the efficacy of OV-mOX40L. They analyzed the tumor microenvironment (TME) and performed various assays to assess T-cell response, tumor growth, and survival.
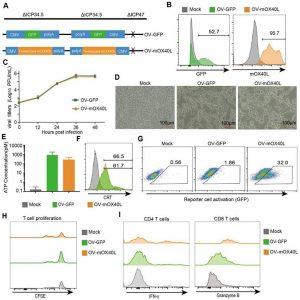
Figure 1 : Generation and characterization of OX40L-armed oncolytic HSV-1. A) The schematic representation of HSV-1 based oncolytic virus encoding murine OX40L or GFP. The trimerized mOX40L was constructed by IL2 signal sequence-Traf2 coiled-coil domain-mOX40L extracellular domain-PDGFR transmembrane domain. B) GFP or membrane-displayed OX40L expression level on KPC cells after infection with OV-GFP or OV-mOX40L. KPC cells were infected with OV-GFP or OV-mOX40L at an MOI of 1. Cells were stained with OX40L antibody after 24 hours and detected by flow cytometry. C) Virus replication in the KPC cells. KPC cells were infected with OV-GFP or OV-mOX40L at an MOI of 0.1, and viral titers were determined using plaque assays at different times post infection. D) Cytopathic effect (CPE) of OV-mOX40L against KPC cells. KPC cells were infected with OV-GFP or OV-mOX40L at an MOI of 1. Cells were observed under the microscope after 24 hours. E-F) ICD of tumor cells was induced by OV-mOX40L. KPC cells were infected with OV-GFP or OV-mOX40L for 48 hours. The released ATP was detected by the ATP detection kit (E) and the cell surface exposure of calreticulin was analyzed by flow cytometry (F). The data for ATP concentration were representative of three independent experiments. G) Effect of OV-mOX40L on Jurkat-NF-κB-GFP-OX40 reporter cells. HEK293FT cells were infected with OV-GFP or OV-mOX40L and cocultured with Jurkat-NF-κB-GFP-OX40 reporter cells, which express GFP upon activation of the OX40-OX40L signaling pathway. The GFP expression of the reporter cells was analyzed by flow cytometry. H) Effect of OV-mOX40L on T cell proliferation. CFSE-labeled T cells were cocultured with OV-infected KPC cells in 96-well plate precoated with anti-CD3 antibody for three days. The proliferation of T cells, which exhibited decreased CFSE fluorescence intensity, was detected by flow cytometry. I) Effect of OV-mOX40L on T cell activation. T cells were cocultured with OV-infected KPC cells in 48-well plate precoated with anti-CD3 antibody for 24 hours. The activation markers IFN-γ and granzyme B of T cells were detected by flow cytometry.
The results showed that OV-mOX40L effectively delayed tumor growth and extended survival time in the mouse model compared to the parental oncolytic virus. OV-mOX40L treatment increased T-cell infiltration, promoted an activated state in the TME, remodeled the stromal matrix, and enhanced the T-cell response. The study demonstrates that OX40L-armed OV therapy can boost T-cell
Identification of Major Signaling Changes after OV-mOX40L Therapy
The paper analyzed the effect of OV-mOX40L therapy in pancreatic cancer in mice. They identified significantly different signaling pathways between the PBS and OV-mOX40L groups. The IL6:IL6R/gp130 and PDCD1:PD-L1 signaling networks were inferred among different cell populations. The upregulation of these pathways could negatively impact anti-tumor immunity, suggesting the potential for combination therapy with neutralizing antibodies against IL6 or PD-L1 pathways.
Tumor-Infiltrating Immune Cells and Fibroblasts after Oncolytic Virus Treatment
The study analyzed tumor-infiltrating immune cells and cancer-associated fibroblasts (CAFs) after treatment with oncolytic viruses. OV-mOX40L treatment reduced Treg counts, increased the proportion of proliferated CD4 T cells, and activated CD8+ T cells while mitigating CD8+ T cell exhaustion. The treatment also induced a shift in macrophages from anti-inflammatory to pro-inflammatory and decreased the number of fibroblast active protein (FAP)-expressing CAFs.
Cell-Cell Communications in Pancreatic Cancer
The paper performed unbiased cell-cell interaction inference analysis to analyze the global alterations in cell-cell communications in pancreatic cancer. They identified the signaling communication pathways with the highest interaction strength ratios in the PBS and OV-mOX40L groups. The OV-mOX40L treatment showed increased intercellular IL6 signaling interactions among macrophages, DCs, Tregs, and iCAFs, as well as enhanced PD-L1 pathway interactions among immune cells.
The study demonstrated that OV-mOX40L treatment reinvigorated intra-tumoral immune cells, remodeled the tumor microenvironment, and enhanced the T cell response in pancreatic cancer. The treatment significantly prolonged the survival of PDAC mice, either as a monotherapy or in combination with synergistic antibodies. The results suggest a multimodal therapeutic strategy for pancreatic cancer treatment, targeting pathways such as VEGF:Flt1/Flt4/Kdr, PDCD1:PD-L1, and IL6:IL6R/gp130.
Journal article: Liu, S., et al., 2023. OX40L-Armed Oncolytic Virus Boosts T-cell Response and Remodels Tumor Microenvironment for Pancreatic Cancer Treatment. Theranostics.
Summary by Dounia Chraa










