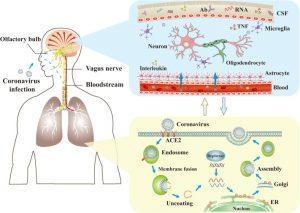
The mechanisms of coronaviruses infections and neurological damage caused by coronaviruses. The coronaviruses can cause nerve damage through direct infection pathways (blood circulation pathways and neuronal pathways), hypoxia, immune injury, ACE2, and other mechanisms. Meanwhile, the coronaviruses have detrimental effects to attack the lung tissue, and causes a series of lung lesions such as hypoxia. Furthermore, the coronaviruses can enter the nervous system directly through the olfactory nerve, and also enter the nervous system through blood circulation and neuronal pathways, resulting in neurological disorders. Ab: antibody; ACE2: angiotensin-converting enzyme 2; CSF: cerebrospinal fluid; ER: endoplasmic reticulum; TNF: tumor necrosis factor. (Source: Wu et al., 2020)
A brief summary of potential immunologic mechanisms underlying COVID-19-related neuropsychiatric outcomes.
As the world continues to make significant progress around SARS-CoV-2 vaccination research and effective therapies for severe COVID-19, significantly less attention has been given to the acute neuropsychiatric sequelae of SARS-CoV-2 infection borne out of recent reports [1, 2] and its potential to become a significant global disease burden post-COVID-19. Acute and sub-acute neuropsychiatric manifestations that have been documented in previous viral pandemics (H1N1, SARS-CoV-1, MERS-CoV) have also been reported in COVID-19 cases, and to-date, include: anosmia, ageusia, encephalopathy, delirium, and cerebrovascular complications. Although the rise of more chronic neuropsychiatric sequelae, such as depression, anxiety, and insomnia, have often coincided with previous coronavirus outbreaks, the immunologic mechanisms for which this occurs is still unclear and has yet to be documented for the current COVID-19 pandemic.
A recent review published by Troyer et al. highlights the potential immunologic mechanisms underlying neuropsychiatric links to SARS-CoV-2 infection, drawing from experiences with past CoV pandemics and current evidence [3]. One mechanism is through ACE2-receptor mediated cytokine network dysregulation. SARS-CoV-2 binds to the ACE2 receptor, expressed on neurons, glia, and gut epithelial cells, indicating a direct pathway for CNS and gastrointestinal manifestations. In one experimental study by Netland et al., intranasal inoculation of SARS-CoV-1 in ACE2 transgenic mice resulted in neuronal death and upregulation of TNF-alpha, IL-1-beta, and IL-6 by astrocytes, suggesting its role for the development of subsequent encephalopathies via CNS-localized inflammation [4].
However, as with many other infections, SARS-COV-2 does not necessarily need to directly interact with the CNS to initiate a neuropsychiatric response. Host anti-SARS-CoV-2 responses may make the blood-brain-interface more permeable upon inflammation and psychological stress, leading to potential evasion by peripheral myeloid cells and subsequent propagation of neuro-inflammation via pro-inflammatory cytokines and microglial activation [5].
Another speculative immunologic mechanism of COVID-19-associated neuropsychiatric sequelae is through gut microbial translocation, known as the gut-brain axis (e.g. vagus nerve) (check previous blog post: https://www.immunopaedia.org.za/breaking-news/further-support-of-the-emerging-immunologic-hypothesis-in-mood-and-eating-disorders/). Recent reports indicate that up to almost 40% of COVID-19 patients present with gastrointestinal symptoms and that viral shedding in COVID-19 patient feces is a persistent phenomenon (>5 weeks post-infection), suggesting that changes in the gut-brain-axis are mechanistically feasible, but speculative at this time.
Although scarce, the evidence mentioned thus far further emphasizes the need to study the psychoneuroimmunological aspect to SARS-CoV-2 infection, which is critical in managing the potentially long-term and chronic effects of COVID-19 on health care systems and societies globally. Moreover, it pushes for more attention on the neurological and psychological fallout of individuals affects by this pandemic, which raises awareness around the appropriate care planning that needs to take place for their support.
References:
- Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., … & Miao, X. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA neurology, 77(6), 683-690.
- Wu, Y., Xu, X., Chen, Z., Duan, J., Hashimoto, K., Yang, L., … & Yang, C. (2020). Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain, behavior, and immunity.
- Troyer, E. A., Kohn, J. N., & Hong, S. (2020). Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, behavior, and immunity.
- Netland, J., Meyerholz, D. K., Moore, S., Cassell, M., & Perlman, S. (2008). Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. Journal of virology, 82(15), 7264-7275.
- Lavi, E., & Cong, L. (2020). Type I astrocytes and microglia induce a cytokine response in an encephalitic murine coronavirus infection. Experimental and Molecular Pathology, 104474.
Article by Bryan Gascon & Osana Ratnaharan










