- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final outcome
- References
- Evaluation - Questions & answers
- MCQ
Patient Presentation
A 33 year old female presents to the medical out patient department with a moderately severe headache, sudden onset of weakness in the right hand, numbness of the right side of her face and slurring her words. The symptoms had started about 3 hours earlier.
Acknowledgement
This case study was kindly provided by Dr Monica Mercer, MBChB from Immunopaedia
Partnership
We have partnered with The International Union of Basic and Clinical Pharmacology (IUPHAR) to bring you in-depth information about drugs and pharmacology with links to the Guide to ImmunoPharmacology.
History
History was obtained from the patient’s family, husband and mother.
According to the family the patient had woken in the morning complaining of a headache and blurred vision. She decided to lie down again and fell asleep for about an hour. On waking she was unable to speak properly, slurring her words and indicating that her right hand was weak and the right side of her face felt numb.
She had been unwell for the last week, complaining of flu like symptoms; headache, fever, some abdominal pain, a rash and tender gums, which had bled a few times. She had not seen a doctor during this time, and was self medicating with paracetamol.
Past medical history
- Previously well, no chronic disease, no previous admissions.
Past Surgical History
- No history of any surgical procedures.
Family History
- Mother has hypertension and is on treatment from her local clinic.
- Father died in a car accident several years earlier, was suspected to have hypertension but not on treatment.
- Three siblings, all healthy
Medications
- No chronic medication
- Currently using paracetamol 1g tds
Allergies
- Nil
Social history
- Employed in a hair salon as a hairdressers assistant.
- Non smoker
- No alcohol consumption
Travel History
- Nil
Differential Diagnosis
- A transient ischaemic attack (TIA)
- Reversible ischaemic neurologic deficit (RIND)
- Ischaemic stroke
- Haemorrhagic stroke
- Disseminated Intravascular Coagulation
- SLE
- Idiopathic thrombocytopenic purpura
- Thrombotic thrombocytopenic purpura
Patient awake, alert, orientated to person, place and time, but ill looking.
Thin but not wasted
Vitals
- Fever, 38°C
- Blood pressure 166/104
- Heart rate 98bpm
- Respiratory rate 16
General
- Mild pallor
- Shotty cervical lymphadenopathy
- Mild jaundice noted in the sclera
- No oedema
- No signs of dehydration
Respiratory
- Trachea centrally located.
- Chest clear on auscultation
Cardiovascular
- No raised JVP
- Normally placed apex beat
- S1 and S2 heart sounds present, no murmurs.
- No abnormalities detected
Abdomen
- Not distended
- Soft, but mildly tender on palpation
- No organomegally
- Bowel sounds present
Neurological*
- Higher function intact
- Dysarthria
- Right sided facial palsy
- Normal gait
- Power in right upper limb 3/5, all other limbs 5/5
- Tone normal globally
- Reflexes 2/4 for both upper and lower limbs
- No cerebellar signs
Dermatological/Haematological
- Petechial rash noted on the upper trunk and legs.
- Multiple petechial haemorrhages were noted on the palate and some gingival bleeding
* of note, neurological symptoms completely resolved shortly after initial examination. They returned 5 hours later, and resolved again about an hour later.
Investigations
| Examination | Value | Limits |
|---|---|---|
| WBC | 10.7 | (4-12 x109/L) |
| HB | 8 | (12.1-15.2 g/L) |
| Platelets | 18 | (140-450 x109/L) |
| Blood film | Fragmented RBC in peripheral blood (schistocytes) | |
| NA | 138 | (135-147 mmol/L) |
| K | 4.5999999999999996 | (3.3-5.0 mmol/L) |
| CL | 100 | (99-103 µmol/L) |
| C02 | 26 | (18-29 mmol/L) |
| Urea | 5.2 | (2.5-6.4 mmol/L) |
| Creatinine | 101 | (62-115 mmol/L) |
| Total protein | 65 | (60-80g/L) |
| Albumin | 38 | (35-50g/L) |
| TBIL | 14 | (µmol/L) |
| DIR BIL | 9 | (0-4 µmol/L |
| Alp | 61 | (30 - 150 U/L) |
| GGT | 34 | (11 - 51 U/L) |
| ALT | 23 | (5 - 35 U/L) |
| AST | 80 | (5 - 35 U/L) |
| LDH | 1250 | (30 - 150 U/L) |
| HIV Elisa | positive | |
| CD4 count | 376 | |
| Urine sample | +++ blood | |
| +++ protein |
Imaging
CT Brain was normal showing no signs of intracranial or subarachnoid haemorrhage and no signs of ischaemic changes.
Discussion
The patient in our case study was diagnosed with thrombotic thrombocytopaenic purpura (TTP). TTP is a pentad of neurological symptoms, pyrexia, renal dysfunction and microangiopathic haemolytic anaemia with thrombocytopaenia. The platelet microthrombi which form in the microcirculation are responsible for most symptoms seen in this condition due to resulting organ ischaemia. The endothelia of the kidneys, brain, heart, pancreas, spleen, and adrenal glands are particularly vulnerable to TTP. While the liver, lungs, gastrointestinal tract, gallbladder, skeletal muscles, retina, pituitary gland, ovaries, uterus,and testes are affected to a lesser extent.
To better understand the pathophysiology of TTP it is important to first understand platelet function, the role of von Willebrand Factor (vWF) and the proteolytic enzyme ADAMTS13, when endothelium is injured.
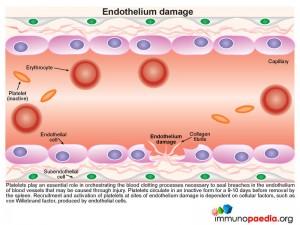
Endothelium Damage
During this process platelets play an essential role in orchestrating the blood clotting processes necessary to seal breaches in damaged blood vessels. Recruitment and activation of platelets at sites of endothelium damage is dependent on vWF, which is synthesized by endothelial cells. The endothelial cells located near the site of damage respond by producing vWF which is secreted in the form of large multimeric chains.
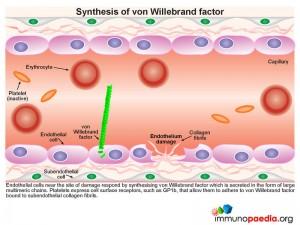 Synthesis of von Willebrand Factor
Synthesis of von Willebrand Factor
A plasma-derived proteolytic enzyme, ADAMTS13, cleaves the multimeric chains of vWF into smaller fragments. The enzyme recognizes specific peptide cleavage sites on the multimeric chains that become exposed when the tightly folded protein elongates due to high shearing forces of small blood vessels. The cleaved fragments of vWF bind to the exposed subendothelial collagen.
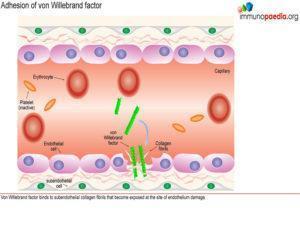
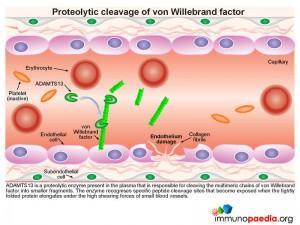 Platelets express cell surface receptors, such asGP1b, that allows them to detect and adhere to vWF bound to collagen fibrils. In addition platelets express cell surface receptors, such as GPIIb/IIIa, that mediates platelet binding to other platelets via fibrinogen intermediates. These factors contribute to recruitment of platelets to the site of endothelium damage and platelet aggregation that forms a seal in the breached endothelium. The platelets also initiates the blood clotting cascade that generates a meshwork of insoluble fibrin.
Platelets express cell surface receptors, such asGP1b, that allows them to detect and adhere to vWF bound to collagen fibrils. In addition platelets express cell surface receptors, such as GPIIb/IIIa, that mediates platelet binding to other platelets via fibrinogen intermediates. These factors contribute to recruitment of platelets to the site of endothelium damage and platelet aggregation that forms a seal in the breached endothelium. The platelets also initiates the blood clotting cascade that generates a meshwork of insoluble fibrin.
What is thrombotic thrombocytopaenic purpura (TTP)?
 In TTP, the plasma-derived proteolytic enzyme ADAMTS13, that cleaves the large multimeric chains of von Willebrand factor into smaller fragments, is depleted due to the presence of an autoantibody.
In TTP, the plasma-derived proteolytic enzyme ADAMTS13, that cleaves the large multimeric chains of von Willebrand factor into smaller fragments, is depleted due to the presence of an autoantibody.
 Autoantibodies can be produced through molecular mimicry following a humoral response to an infection or by disruption of tolerance to self-antigens. This is observed in autoimmune diseases like SLE or during HIV infection. During HIV infection, there is both disruption of CD4+ T cell regulation and an increased risk of opportunistic infections. There is thus a greater likelihood of autoantibody generation through molecular mimicry. Because in this case, our patient was HIV infected, there was a strong likelihood that the action of ADAMST13 was inhibited, or neutralized, by the formation of autoantibodies.
Autoantibodies can be produced through molecular mimicry following a humoral response to an infection or by disruption of tolerance to self-antigens. This is observed in autoimmune diseases like SLE or during HIV infection. During HIV infection, there is both disruption of CD4+ T cell regulation and an increased risk of opportunistic infections. There is thus a greater likelihood of autoantibody generation through molecular mimicry. Because in this case, our patient was HIV infected, there was a strong likelihood that the action of ADAMST13 was inhibited, or neutralized, by the formation of autoantibodies.
TTP can also occur during pregnancy and may be related to the increased risk of developing autoantibodies when immune responses are polarized towards a Th2 cytokine profile, which favours development of humoral immunity.
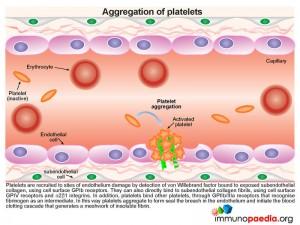
In t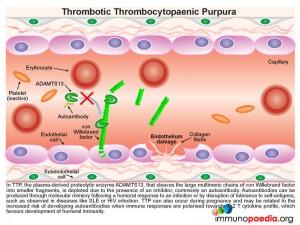 he absence of ADAMTS13 proteolytic activity, the large chains of vWF multimers are increased in the circulation and bind to exposed subendothelial collagen fibrils, initiating recruitment of large numbers of platelets to sites of endothelium damage.
he absence of ADAMTS13 proteolytic activity, the large chains of vWF multimers are increased in the circulation and bind to exposed subendothelial collagen fibrils, initiating recruitment of large numbers of platelets to sites of endothelium damage.
Due to the size and number of these chains excessive numbers of platelets are recruited and activated, resulting in:
- Platelet depletion or thrombocytopaenia
- Impedence of red cells through small blood vessels causing cells to shear with a resulting microangiopathic haemolytic anaemia (MAHA) and organ ischaemia
- Fragments of erythrocytes are visible in blood smears and are known as schistocytes.
Treatment of TTP
The diagnosis of TTP is essentially a clinical diagnosis. Although not all 5 criteria listed in the pentad are always present, life saving treatment is often initiated on the evidence of MAHA alone.
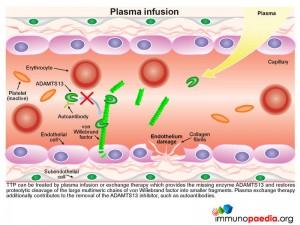
TTP can be treated by plasma infusion or exchange therapy which provides the missing enzyme ADAMTS13 and restores proteolytic cleavage of the large multimeric chains of vWF into smaller fragments, thus avoiding excessive platelet aggregation in small blood vessels. Plasma exchange therapy additionally contributes to the reduction or removal of the autoantibodies to ADAMTS13 by diluting them out.
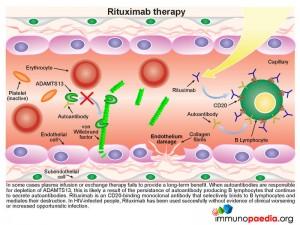
In some cases plasma infusion or exchange therapy fails to provide a long-term benefit. This is found where the inhibition of ADAMTS13 is due to the persistence of autoantibody producing B lymphocytes that are not removed by plasma treatment. In these circumstances treatment with rituximab should be considered. This is a CD20 binding monoclonal antibody that selectively binds to B lymphocytes and mediates their destruction. Removal of the source of the autoantibody, results in restoration of significant ADAMTS13 activity.
Download images for this case
Treatment
As this patient was HIV infected, it was decided that the likely immunosuppressive effects of rituximab could be detrimental and was therefore not used. We suspected that Rituximab would have rendered her lymphopaenic or hypogammaglobulinaemic, or both. However, there has been a recent report that Rituximab was used to treat TTP in HIV-infected people who were refractory to conventional treatment. It was shown to be successful with no evidence of clinical worsening or increased opportunistic infection. Our patient was admitted and underwent plasma exchanges with fresh frozen plasma daily for 12 days, until her symptoms completely resolved.
Download images for this case
Our patient had no further neurological or renal symptoms once treatment was started. Complete remission was achieved after 14 days, and she was discharged within three weeks from admission. We speculate that the successful treatment lead to replacement of the missing enzyme ADAMTS13 and sufficient removal of autoantibodies. This allowed the neutralization of ADAMTS13 proteolytic activity to be alleviated. She was followed-up by the medical out-patient department who subsequently started her on anti-retroviral therapy (ART). She has not presented with any further episodes of TTP and we speculate that successful ART also removed the aetiology behind autoantibody generation.
Download images for this case
References
Ross CL et al. (2009). HIV, thrombotic thrombocytopaenic purpura and rituximab in a violent noncompliant patient. Blood Coagul Fibrinolysis. Mar;20(2):157-60.
Link to Abstract
Scully MA et al. (2009). Berend Houwen Memorial Lecture: ISLH Las Vegas May 2009: the pathogenesis and management of thrombotic microangiopathies., Int J Lab Hematol. Jun;31(3):268-76.
Link to Abstract
Copelovitch L et al. (2008). The thrombotic microangiopathies. Pediatr Nephrol. Oct;23(10):1761-7. Epub 2007 Sep 30. Review.
Link to Abstract
Download images for this case
Evaluation – Questions & answers
What is the diagnosis?
What is TTP?
What is the mechanism of TTP?
In the absence of ADAMTS13 proteolytic activity, the large chains of vWF multimers are increased in the circulation and bind to exposed subendothelial collagen fibrils, initiating recruitment of large numbers of platelets to sites of endothelium damage. Due to the large size of these chains excessive numbers of platelets are recruited, resulting in thrombocytopaenia, partial occlusion of vessels resulting in organ ischaemia and fragmentation of red cells causing microangiopathic haemolytic anaemia (MAHA).
What are the fragments of erythrocytes visible on blood smears called?
How do autoantibodies arise against ADAMTS13?
Autoantibodies may also be generated when there is a disruption of tolerance to self-antigens, such as observed in autoimmune disease like SLE. In HIV infection, autoimmune responses can also occur as a result in dyregulation of infection. This, in turn, leads to the generation of cross-reactive antibodies to self antigens, through a process known as molecular mimicry.
What is the treatment for TTP?
How does this treatment work?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case