- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Case Evaluation – Questions & answers
- MCQ
Patient Presentation
A previously well 17 month old boy presents with a three day history of fever and a skin rash. No accompanying cough or diarrhoea.
Acknowledgements:
Dr Christiaan Scott and Dr Kate Weakley Webb, Paediatric Rheumatology, Red Cross War Memorial Children’s Hospital, University of Cape Town, South Africa.
Partnership
We have partnered with The International Union of Basic and Clinical Pharmacology (IUPHAR) to bring you in-depth information about drugs and pharmacology with links to the Guide to ImmunoPharmacology.
SPONSOR(S):
History
The patient and his family recently moved to South Africa from Burundi (Eastern Africa). His mother reports that prior to this presentation the child has been well. She is unaware of him having any contact with TB or other infectious illnesses.
According to his Road to Health Card he was born full term from a normal vaginal delivery with no complications noted.
He was breast fed for the first 10 months.
He is noted to be HIV unexposed, but no PCR had been done to confirm this.
All growth parameters have been consistent and above the 50thcentile for height and weight.
Past Medical History
- Nothing reported
Past Surgical History
- Nothing reported
Vaccination History
- BCG given at birth
- No other vaccination history available
Family History
- Lives with both parents and three older siblings aged 3, 5 and 8 in a 1 bedroom apartment with all amenities.
Allergies
- None known
Medication
- Nil
Travel History
- Recent road travel from Burundi to South Africa
Differential Diagnosis
Differential diagnosis of an otherwise well child with a rash and fever:
Infections:
Viral
- Measles
- Rubella
- Parvovirus
- Coxsackie
- HHV 7
- Varicella
- Adenovirus
- Rhinovirus
- Influenza
- Parainfluenza
Bacterial
- Scarlet fever
- Meningococcus
- Ricketsia
- Lyme disease
Autoimmune
- Post streptococcal
- Kawasaki disease
- Henloch-Schonlein Purpura
Malignancy
- Leukemia
- Lymphoma
Examination
In Admission:
Appearance
- Ill looking child, alert and awake
- Well nourished
Vitals
- Temperature: 40oC
- Heart rate: 105
- Respiratory rate: 25
General
- Normal weight
- No dehydration
- Warm peripheries
- Cervical and axillary lymphadenopathy
ENT
- Mild pharyngitis
Chest
- Chest clear
Cardiovascular
- Mild tachycardia
Abdomen
- Not distended
- Soft, no generalised tenderness
- No organomegally
- Bowel sounds present
Neurological
- Normal level of consciousness
- Alert and co-operative
Dermatological
- Generalised maculo popular rash
Child was admitted (see investigations), and discharged after 2 days on Amoxil, tobe followed up at out patient clinic in 48 hours to have Mantoux test read
Nine days after initial presentation the patient again presents to hospital
Five days earlier, the patient was started on TB treatment following a positive Mantoux test and a suspicious looking chest X-ray. He now presents again with a fever and 7-day swelling of the face.
On Examination
- Ill looking child
- Temperature 39oC
- Generalized lymphadenopathy
- Lips swollen, drooling, red tongue and throat.
- Perioral peeling
- Periorbital dryness anderythema
- Warm swollen hands and feet, with painless palpable effusions over the ankles
- Swollen, red BCG vaccination site and red mantoux mark
- Peeling rash on trunk, neck and axillae
Patient was re-admitted, an echocardiogram was performed which was normal and he was treated with 2g polygam, paracetomol and 50mg aspirin daily (see investigations).
He improved and was discharged
10 days later the patient returned to hospital
Although he no longer had a fever his mother was concerned about the persistent rash all over his body and a recurrent swelling of his hands and feet, which had become painful, preventing the child from walking.
On Examination
- Ill looking child with marked irritability
- Apyrexial
- Swollen hands, feet and ankles, with +dactylitis.
- Generalized lymphadenopathy
- Generalized erythematous macular rash
Patient was readmitted
No repeat polygam given as patient had responded to the first dose
Patient was kept on 20mg daily aspirin, Ibuprofen for pain and sucralfate
TB treatment was continued
(see investigations)
He was discharged home on Aspirin, pain medication and a temperature chart
One month later, patient was seen as an outpatient for a check up
The home temperature chart revealed no fever.
On Examination
- Apyrexial
- Lymphadenopathy had resolved
- Hands and feet were still swelling occasionally, but he was able to walk. Some residual desquamation was noted on the hands
- No joint involvement
- BCG and Mantoux sites were normal
- Pain medication was stopped
- TB medication was continued for the full course
Patient was booked for a 3-month repeat Echo
(see investigations)
Investigations
On Presentation
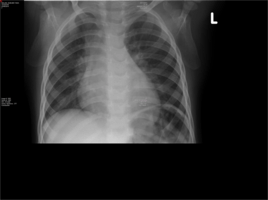
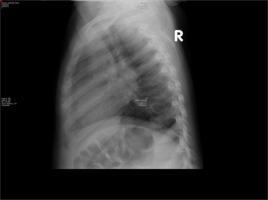
- Chest X-Ray- Widened mediastinum and hilar lymphadenopathy
- Mantoux performed based on CXR finding. Area of induration at 48 hours was >15mm and considered positive.
9 days later
- ESR 97
- HIV negative
- ASOT negative
- antiDNAse negative
- TB gastric washings, repeated on three occasions- all negative
- Echocardiogram-normal
2 weeks later
- Repeat echocardiogram- normal
- ESR 75, CRP 15
- ANA negative
- Sickle test negative
Discussion
Introduction
This case focuses on an acutely ill 17-month-old baby boy who presented to a paediatric hospital in South Africa having recently moved from Burundi in eastern Africa. His initial presentation was of a rash, fever and pharyngitis, for which he was treated with oral antibiotics. A routine TB work up was done. Based on the hilar lymphadenopathy on chest X-ray, and a positive Mantouxtest, he was started on TB treatment, despite not having a definitive microbiological diagnosis. As the paediatric hospital lies in an area with high TB prevalence, treatment is standard protocol. However within a few short days his signs and symptoms worsened.
Background
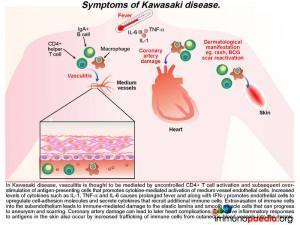
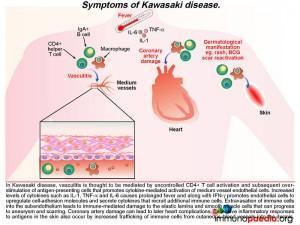
A diagnosis of Kawasaki Disease (KD) was made and the following discussion will focus on this acute multisystem vasculiticsyndrome of unknown etiology. KD is seen predominantly in infants andchildren younger than 5 years of age and the disease occurs globally, having been first diagnosed in Japan in the 1960s.
KD is characterized by prolonged fever, conjunctivitis, diffuse mucosal inflammation, polymorphous skin rashes, indurative oedema of the hands and feet with an associated peeling of finger tips and non-suppurative lymphadenopathy.The most severe complication in KD is that of acute coronary syndrome, including myocardial infarction and coronary artery aneurysms,which ispathognomonic when identified in the setting of a compatible febrile illness.To date, there are no specific diagnostic tests for KD,instead the diagnosis is made clinically by the presence of fever for five days and 4 out of 5 of the following criteria:
- Non-purulentbilateral conjunctivitis
- Cervical lymphadenopathy of nodes >1.5 cm
- Polymorphous skin rashes
- Strawberry tongue, fissured lips and/or diffuse erythema of the oropharynx
- Oedema of the palms and soles and desquamation of the fingertips.
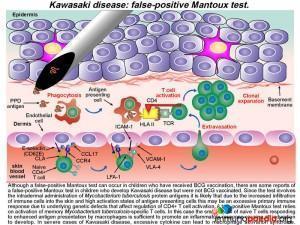
It has also been noted that in countries where newborn babies receive Bacillus Calmette-Guerin (BCG) vaccination, KD canbe associated with erythematousinduration or even ulceration of BCG scars in one-third of cases. The patient in this case study had received BCG at birth and during the course of his illness presented with BCG scar reactivation and a positive Mantoux test with a reading of >15mm.
Although the aetiology of KD is not well understood, with the aid of our case study and graphics we will explore the ways in which immune-mediated destruction of the vascular system occurs following the introduction of a yet to be identified immunogenic agent. We will also discuss the role that genetics has to play in this disease by looking specifically at two of the genes that have been implicated. We will also explain how this vasculitic syndrome may be connected to BCG scar reactivation and a positive Mantoux test in the absence of TB infection.
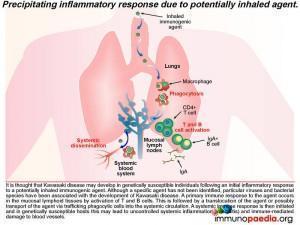
To better understand Kawasaki disease, let’s first look at the precipitating inflammatory response
It is thought that KD may develop in genetically susceptible individuals following an initial inflammatory response to a potentially inhaled immunogenic agent. Although no specific agent has been identified, prior infection by one of a number of viruses and bacterial species has been associated with the development of KD. These include EBV, HIV, measles, Staph. aureus, Strep. pyogenes and Mycoplasmapneumoniae, to name a few. A primary immune response to the agent occurs in the mucosal lymphoid tissues by activation of T and B cells, which is then thought to be followed by a translocation of the agent or possibly transport of the agent via trafficking phagocytic cells into the systemic circulation. A systemic immune response is then initiated and in genetically susceptible hosts this may lead to the uncontrolled systemic inflammation and immune-mediated damage of blood vessels or vasculitis.
What are the signs of this developing vasculitis in Kawasaki Disease?
The ensuing vasculitisis thought to be mediated by uncontrolled activation of CD4+ T cells and antigen-presenting cells and subsequent cytokine-mediated activation of medium vessel endothelial cells. The increased levels of cytokines such as IL-1,TNF-alpha and IL-6 cause the prolonged fever as seen in this patient. Along with IFN-g, these pro-inflammatory cytokines promote endothelial cells to up-regulate cell-adhesion molecules and secrete cytokines that recruit additional immune cells. Extravasation of immune cells into the subendothelium leads to immune-mediated damage to the elastic lamina and smooth muscle cells. This weakens the blood vessel wall that in time can progress to aneurysm formation and scarring. When coronary arteries are involved it can result in ischaemic heart complications (not present in this patient). Excessive inflammatory responses to antigens in the skin also occur because there is increased trafficking of immune cells from cutaneous blood vessels into the dermis. This can result in a persistent generalized rash and BCG scar activation, as seen during the course of illness in our patient.
As we know KD is a multisystem vasculitic syndrome. In the acute stage, numerous immunologic factors including CD4+ T cell activation, cytokine production and enhanced adhesion molecule expression by endothelial cells mediatesthe vasculitis. These various processes are further discussed here.
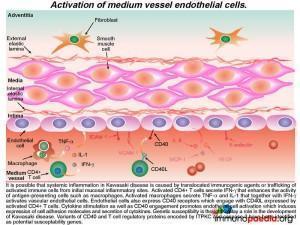
Lets look more closely at the activation of medium vessel endothelial cells
The activated CD4+ T cells secrete IFN-g that enhances the activity of phagocytic cells such as macrophages. Activated macrophages secrete TNF-a and IL-1 that together with IFN-g activates vascular endothelial cells. Endothelial cells also express CD40 receptors, which engage with CD40L (CD154) present on the surface of activated CD4+ T cells.
The resulting tethered CD4+ cells to the vascular endothelium secrete cytokines, which further activates the endothelial cells and induces them to express further cell adhesion molecules (ICAM-1, VCAM-1 and E-selectin) and secreteIL-1, TNF-a and IFN-g. Genetic susceptibility is thought to play a role in the development of Kawasaki disease. In particular, polymorphisms in two genes encoding the T cell regulatory protein ITPKC and Caspase-3. We will uncover these specific events towards the end of our discussion.
Activated endothelial cells also secrete IL-6 in sufficient amounts to promote fever by acting on the hypothalamus and to induce the liver to synthesize acute phase proteins (such as CRP). MCP-1 is a chemo-attractant for monocytes and VEGF promotes vascular permeability and enhances extravasation of immune cells into the sub-endothelium.
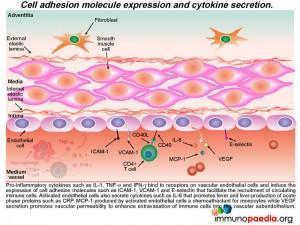
Recruitment of monocytes and neutrophils to the endothelium
Activated monocytes, recruited to the sub-endothelium by interacting with the adhesion molecules (ICAM-1 and VCAM-1) on endothelial cells, mature into macrophages and secrete pro-inflammatory cytokines such as IL- 1, IL-6 and TNF-a.
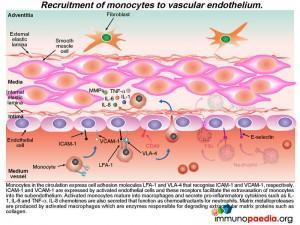 Neutrophils are also recruited to the activated endothelium by recognition of E-selectin that binds to ESL-1. Neutrophil recruitment is enhanced by IL-8, a chemokine released by activated monocte / macrophages Neutrophils then extravasate into the subendothelial tissue where they secrete elastase, a serine protease that degrades elastin of connective tissue. Activated monocytes / macrophages release matrix metalloproteases, protease enzymes that degrade extracellular matrix proteins, such as collagen.How this damages the subendothelium is discussed in greater detail a little later.
Neutrophils are also recruited to the activated endothelium by recognition of E-selectin that binds to ESL-1. Neutrophil recruitment is enhanced by IL-8, a chemokine released by activated monocte / macrophages Neutrophils then extravasate into the subendothelial tissue where they secrete elastase, a serine protease that degrades elastin of connective tissue. Activated monocytes / macrophages release matrix metalloproteases, protease enzymes that degrade extracellular matrix proteins, such as collagen.How this damages the subendothelium is discussed in greater detail a little later.
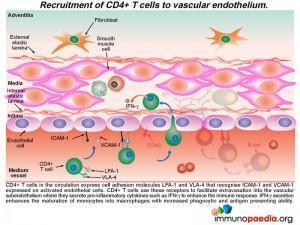
The recruitment of CD4+ T cells to the endothelium occurs in a similar way
Additional CD4+ T cells in the circulation that express cell adhesion molecules LFA-1 and VLA-4 bind to ICAM-1 and VCAM-1 that are expressed on activated endothelial cells. This CD4+ T cell interactionfacilitates extravasation into the subendotheliumwhere they further secrete IFN-g, which in turn, enhances the maturation of monocytes into macrophages with increased phagocytic and antigen presenting abilities.
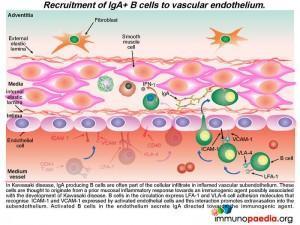
Recruitment of IgA-secreting B cells to the endothelium
In KD, IgA producing B cells are also often part of the cellular infiltrate in the inflamed subendothelium. These are thought to originate from an earlier mucosal inflammatory response to the immunogenic agent, the probable trigger leading to KD. B cells in the circulation express LFA-1 and VLA-4 cell adhesion molecules that recognise ICAM-1 and VCAM-1 expressed by 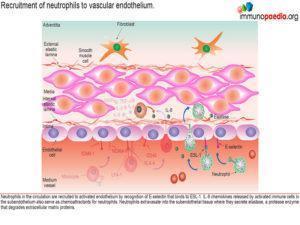 activated endothelial cells and this interaction promotes B cell extravasation into the subendothelium. Activated B cells in the endothelium secrete IgA.
activated endothelial cells and this interaction promotes B cell extravasation into the subendothelium. Activated B cells in the endothelium secrete IgA.
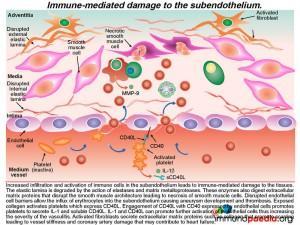
Immune-mediated damage leading to aneurysm and scarring
The result of this mass movement of activated cells and the effects of cytokines is damage and dissolution of the subendothelium tissues. The elastic lamina is degraded by the action of elastases and matrix metalloproteases and these enzymes also digest extracellular matrix proteins that disrupt the smooth muscle architecture resulting in necrosis of smooth muscle cells. Disrupted endothelial cell barriers allow the influx of erythrocytes into the subendothelium causing aneurysm and the development of thrombosis.
The exposed collagen activates platelets, which express CD40L(CD154). The engagement of CD40L with CD40 expressed by endothelial cells results in platelets secreting IL-1 and soluble CD40L.The release of both these solutes causes further activation of endothelial cells and severe inflammation. At the same time activated fibroblasts secrete extracellular matrix proteins, such as collagen, that promotes scarring which results in vessel stiffness. When this occurs in coronary arteries, the damage may contribute to heart disease.
What leads to skin inflammation?
Alongside the well-documented vasculitis, skin inflammation is also often observed in patients with KD. This is seen predominantly as a polymorphous skin rash and is one of the diagnostic criteria for the disease. It is likely that the generalized skin involvement is a consequence of increased infiltration of immune cells through activated endothelium of cutaneous blood vessels into the dermis where an immune response to skin antigens can be mounted. This inflammatory response is excessive due the dysregulation of CD4+ T cells, probably leading to a predominance of Th17 cells, and the high activation state of antigen-presenting cells. It has been noted that in regions where BCG vaccination is in use, up to a third of patients who succumb to KDalso have a reactivation of their BCG scar, along with the usual skin rash.
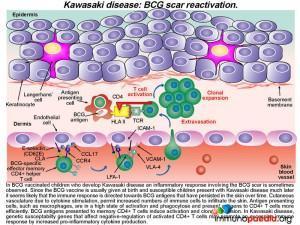
What is the association between Kawasaki disease and BCG scar reactivation?
Since BCG vaccination is usually given at birth and susceptible children show clinical evidence of KD months to years later, it seems likely that the immune response is directed towards antigens present in the vaccine that have persisted in the skin over time. Due to cytokine stimulation, increased numbers of T cells and antigen presenting cells infiltrate the skin (via cutaneous blood vessels) and memory BCG-specific CD4+ T cells become re-activated and clonally expand in numbers. In KD, genetic susceptibility genes that affect negative-regulation of activated CD4+ T cells may promote an excessive immune response by increased pro-inflammatory cytokine production.
In our case study, the patient had both a reactivation of his BCG scar and a (presumed) false positive Mantoux test. While it is well recognized that children who have received BCG vaccination can sometimes have a false-positive Mantoux test due to cross-reactive immune responses to Mycobacterium tuberculosis antigens, there are also some reports of a false-positive Mantoux test in children with KD who were never BCG vaccinated. The Mantoux test involves intradermal administration of Mycobacterium tuberculosis protein antigens. It is likely that a combination of underlying genetic defects that affect negative regulation of CD4+ T cells, an increased infiltration of CD4+ T cells into the skin and the high activation state of antigen presenting cells all lead to an excessive localized immune response in patients with KD. A true-positive Mantoux test relies on activation of memory Mycobacterium tuberculosis-specific T cells, but in this case, a small pool of naïve CD4+ T cells responding to enhanced antigen presentation may be sufficient to promote an inflammatory response causing an induration to develop. In severe cases of KD, macrophage activation syndrome may even be present, due to the highly-activated state of macrophages.
Along with the immune mediated causes for Kawasaki Disease, as discussed, we will also discuss how a genetic predisposition in the patient together with immune dysregulation plays an important role in susceptibility to KD. We explain this using our graphics below.
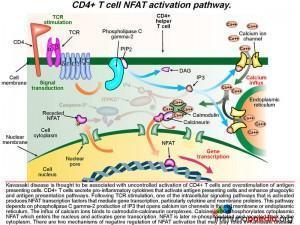
A detailed look at CD4+ T cell NFAT activation pathway
As discussed above, KD is associated with uncontrolled activation of CD4+ T cells and over-stimulation of antigen presenting cells. Following T cell receptor (TCR) stimulation after engagement with the MHC class II-peptide complex on the surface of macrophages, one of the intracellular signaling pathways that is activated results in the translocation to the nucleus of NFAT transcription factors that mediate gene transcription. This pathway depends on phospholipase C gamma-2 production of IP3 that opens calcium ion channels in the cell membrane or in the endoplasmic reticulum. Calcium ions bind to calmodulin-calcineurin complexes. Calcineurin dephosphorylates cytoplasmic NFAT, which enters the nucleus and activates gene transcription, such as cytokines and membrane proteins. NFAT is later re-phosphorylated and recycled back to the cytoplasm. There are two mechanisms of negative regulation of NFAT activation that may play a role in Kawasaki disease.
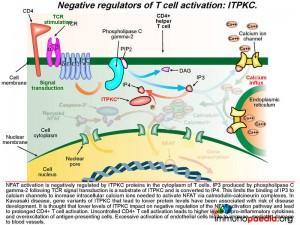
1) Negative regulators of T cell activation: ITPKC
NFAT activation is negatively regulated by ITPKC proteins in the cytoplasm of T cells. IP3 produced by phospholipase C gamma-2 following TCR signal transduction is a substrate of ITPKC and is converted to IP4, which inactivates it. This limits the binding of IP3 to calcium channels to increase intracellular calcium ions needed to activate NFAT via calmodulin-calcineurin complexes. In Kawasaki Disease, gene variants of ITPKC that lead to lower protein levels have been associated with risk of disease development. It is thought that lower levels of ITPKC leads to longer persistence of IP3 following TCR signaling and prolonged activation of NFAT. This results in prolonged and uncontrolled CD4+ T cell activation resulting in release of higher levels of pro-inflammatory cytokines and over-excitation of antigen-presenting cells. Excessive activation of endothelial cells leads to immune-mediated damage to blood vessels that have been described.
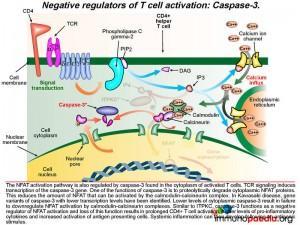
2) Negative regulators of T cell activation: Caspase-3
The NFAT activation pathway is also regulated by caspase-3 found in the cytoplasm of activated T cells. TCR signaling induces transcription of the caspase-3 gene. One of the functions of caspase-3 is to proteolytically degrade cytoplasmic NFAT proteins.
This reduces the amount of NFAT that can be activated by the calmodulin-calcineurin complex. In Kawasaki Disease, gene variants of caspase-3 have been associated with lower transcription levels. Lower levels of cytoplasmic caspase-3 activity leads to increased levels of cytoplasmic NFAT and prolonged CD4+ T cell activation, higher levels of pro-inflammatory cytokines and increased activation of antigen presenting cells. Systemic inflammation can then develop following an antigenic stimulus.
In summary
Although the aetiology of Kawasaki Disease remains largely undetermined, understanding the immunology and genetics behind the mechanisms leading to this condition have been identified and provides insights into our patient’s presentation. Thus, in light of our better understanding of this disease it is most likely that this patient was in fact TB negative at presentation. However due to the high incidence of TB in this geographic location, along with his suspicious clinical and laboratory findings and positive Mantoux test, it remained prudent to provide standard TB medication to this patient.
Download images for this case
Treatment
Patient was treated for 6 months on standard TB treatment
Five days after starting TB treatment, the patient received the standard Kawasaki Disease regimen – 2g polygam, paracetamol and 50mg aspirin daily.
Polygam was not repeated.
Aspirin was continued at a lower dose, 20mg daily, and ibuprofen was given for pain until all symptoms resolved.
Download images for this case
Final Outcome
Skin rash and joint involvement resolved
At 3 months, echocardiogram was repeated and was normal
Download images for this case
References
Cassidy, Petty et al. (2011) Textbook of Pediatric Rheumatology. (Sixth edition). Elsevier Inc ISBN: 978-1-4160-6581-4
Rodó X, Ballester J, Cayan D (2011). Association of Kawasaki disease with tropospheric wind patterns.Science Reports. Nov 10; 1:152. Epub
Lee KY, Rhim JW, Kang JH (2012). Kawasaki disease: laboratory findings and an immunopathogenesis on the premise of a “protein homeostasis system”. Yonsei Med J.Mar;53(2):262-75.
Jia S, Li C, Wang G, Yang J, Zu Y (2010). The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin Exp Immunol. Oct;162(1):131-7. doi: 10.1111/j.1365-2249.2010.04236.x.
Onouchi Y, Ozaki K, Burns JC et al.(2010). A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. Mar 25;44(5):517-21.
Lee YC, Kuo HC, Chang JS et al. (2012). Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat Genet. Mar 25;44(5):522-5.
Scuccimarri R.(2012). Kawasaki disease.,PediatrClin North Am. Apr;59(2):425-45.
Takahashi K, Oharaseki T, Yokouchi Y (2011) Pathogenesis of Kawasaki disease. Clin Exp Immunol. May;164Suppl 1:20-2.
Alexoudi I, Kanakis M, Kapsimali V, Vaiopoulos G (2011). Kawasaki disease: current aspects on aetiopathogenesis and therapeutic management. Autoimmun Rev. Jul;10(9):544-7.
Download images for this case
Evaluation – Questions & answers
What was the final diagnosis?
What is the primary cause of long term morbidity and mortality in Kawasaki Disease?
Which cytokines are known to cause the prolonged fever present in Kawasaki disease?
What are thought to be the precipitating events of the medium vessel vasculitis seen in Kawasaki disease?
Which genetic variants are thought to play a role in susceptibility to Kawasaki Disease?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points- Online Quiz
Download images for this case