- Patient presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final outcome
- References
- Evaluation - Questions & answers
- MCQs
Patient presentation
HIV positive 7 year old female, classified as WHO Stage III. She has been on antiretrovirals for 9 months. She presents with a moderate pyrexia and severe weakness and is reported to have dark urine and pale stools.
Acknowledgement
This case study was kindly provided by the Wits Paeds HIV Clinics.
History
At age 6 years the child was brought into the clinic by her grandmother with unexplained persistent diarrhoea which had lasted for more then 2 weeks. Her grandmother also complained that she did not seem to be growing despite the family always having enough to eat. The child was admitted to hospital which resulted in a 2 month stay.
At this time she was assessed clinically as WHO stage III based on the duration of her diarrhoea, failure to gain weight and the prolonged hospital admission.
After discussing treatment and counseling with her grandmother, the child was prescribed highly active antiretroviral therapy (HAART) – 3TC, d4T and efavirenz (EFV), cotrimoxazole and multivitamins. During the first few days of starting HAART she developed a brief self-resolving rash. Other then this initial incident she has responded well to her treatment with no reported side effects or adverse events.
She has no history of TB or liver disease.
Differential Diagnosis
- Acute hepatitis- prodrome phase of malaise, nausea, vomiting and fever. Followed by the appearance of dark urine and jaundice and tender hepatomegaly.
- Epstein Barr Virus (EBV)- typically present with fatigue, fever, lymphadenopathy and pharyngitis. Hepatomegaly and jaundice occur less commonly.
- Cytomegalovirus (CMV)- febrile illness with hepatitis, jaundice and rash.
- IRIS
- Drug toxicity
Examination
- Axillary temperature 39˚C
- Evidence of jaundice seen in sclera
- Mild discomfort in right upper quadrant and on abdominal examination, hepatomegaly noted.
- No skin rashes or discolouration noted
The remainder of the examination is nil of note
Review of Systems
Pale stools and dark urine
Investigations
| Prior to starting HAART | |
|---|---|
| Baseline CD4 | 311 (13%) |
| ALT | 29 (5-45 U/L) |
| Current Results: | |
| Liver Function Test | |
| Total Bilirubin | 221 (3-18 umol/l) |
| Direct Bilirubin | 193 (0-5 umol/l) |
| ALP | 378 (30-12 U/L) |
| GGT | 149 (5-35 U/L) |
| ALT | 268 (5-45 U/L) |
| AST | 388 (5-45 U/L) |
| INR | 3.68 (1.0) |
| Lactate | 1.5 ( |
| Hepatitis A virus (HAV) | Anti HAV IgM – Negative |
| Hepatitis B virus (HBV) | Anti-HBc IgM and HBsAG – Negative |
| Hepatitis C virus (HBC) | Anti-HCV – Negative |
| EBV | VCA IgM – Negative |
| CMV | CMV IgM and IgG - Negative |
Discussion
Based on the results obtained:
- Acute hepatitis- excluded as all tests negative
- Epstein Barr Virus (EBV)-excluded as result was negative.
- Cytomegalovirus (CMV)- excluded as result was negative
- IRIS – unlikely because symptoms did not occur within the first month of treatment
- Drug toxicity – most likely cause although no liver biopsy was performed
When evaluating the treatment regimen received by the patient, the drug with the greatest potential to induce acute liver failure is co-trimoxazole. Co-trimoxazole, a fixed-dose antibiotic combination of sulfamethoxazole and trimethoprim (TMP-SMZ), is widely prescribed both for bacterial infections and for prophylaxis against opportunistic infections. Despite its broad use, it is associated with a characteristic idiosyncratic hepatotoxicity. This adverse effect often demonstrates features of drug hypersensitivity, suggesting that it results from the metabolism of the drug into a toxic, reactive, or antigenic metabolite.
The typical clinical presentation of TMP-SMZ–induced liver injury involves the acute onset of fever and rash, followed by jaundice, usually within days to weeks after initiating therapy. Reported cases describe symptom onset during the final days of a one-week course or after a latency period of three to four weeks from the start of treatment(“Sulfamethoxazole-Trimethoprim,” 2017). In contrast, the findings in our case differ from this expected pattern, as the patient had been receiving antiretroviral therapy (ART) and TMP-SMZ for nine months before presenting with symptoms.
The pattern of liver injury associated with TMP-SMZ is typically cholestatic or mixed hepatocellular–cholestatic. The severity of injury is variable, ranging from mild, asymptomatic elevations in liver enzymes to severe forms that may progress to acute liver failure.(“Sulfamethoxazole-Trimethoprim,” 2017)
The principles of drug toxicity management (based on WHO guidelines):
- Estimate severity of the effects
- Could drugs other than ARVs cause these symptoms? Evaluate concurrent medications, and establish whether the toxicity is attributable to an ARV or due to other drugs taken at the same time
- Consider other diagnoses, including IRIS and hepatitis infection especially in a child who develops jaundice.
- Manage the adverse event according to the severity:
Maintain regimen for Grade 1 or 2
Substitute regimen for Grade 3
Stop all drugs for Grade 4
| Grade 1 (mild) | Grade 2 (moderate) | Grade 3 (severe) | Grade 4 (life threatening) |
|---|---|---|---|
| Mild Reactions | Moderate reactions | Severe Reactions | Severe life-threatening reactions |
| These reactions are bothersome but they do not require a change in therapy. | Some moderate reactions (e.g. lipodystrophy or peripheral neuropathy) require substitution. For other reactions continue ART as long as is feasible; if the patient does not improve on symptomatic therapy, consider single drug substitution. | Substitute the offending drug without discontinuing ART | Immediately discontinue all ARV drugs and manage the medical event (i.e. symptomatic and supportive therapy) and reintroduce ARV’s using a modified regimen (i.e. substituting another ARV drug for the offending drug) when the patient’s condition is stable. |
| Mild discomfort that does not limit activity. | Some limitation in activity but no medical intervention needed. | Marked limitation in activity. Medical care required. | Extreme limitation in activity. Hospitalization required. |
| Published by International Centre for AIDS care and treatment programs-School of Public Health, Columbia University. |
Treatment
Our patient was assessed with a Grade 4 severity of toxicity and all medication was stopped. This included the ARVs, co-trimoxazole and the multivitamins. All medication was stopped at the same time.
The patient was then continuously observed and liver function results recorded and monitored until baseline values were attained.
ARV first line treatment update.
According to the 2023 Standard Treatment Guidelines for paediatric patients, the regimen to which this patient was initially exposed (lamivudine [3TC] + stavudine [d4T] + efavirenz) is no longer recommended as first line therapy. The updated guidelines designate dolutegravir (DTG)-containing regimens as first-line options (TLD1 and TLD2), with dosing determined by the patient’s weight. In line with these recommendations, the patient would now be initiated on either tenofovir + lamivudine + dolutegravir (TLD1) or abacavir + lamivudine + dolutegravir (TLD2), provided renal function is adequate and irrespective of viral load (Standard Treatment Guidelines and Essential Medicines List for South Africa, 2023)
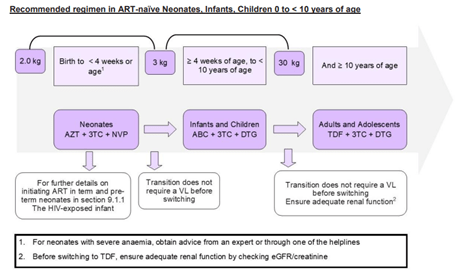
Figure 1 Recommended regimen in ART naive Neonates, Infants, Children 0 to <10 years of age. (Standard Treatment Guidelines and Essential Medicines List for South Africa, 2023)
Final outcome
- Patient was graded as Grade 4 severity of drug toxicity.
- All drugs were stopped at the same time and she made a slow recovery over 3 months.
- All of her liver values normalised and she followed a generally uneventful clinical course.
- Co-trimoxazole toxicity was suspected so she was not re-challenged with the drug.
- Given the evidence for immune restoration based on CD4 recovery PCP prophylaxis was not reinstated.
- She was restarted on d4T, 3TC and EFV without worsening of the hepatotoxicity, up to five months of follow up
References
- Standard Treatment Guidelines and Essential Medicines List for South Africa. (2023). https://www.health.gov.za/nhi-edp-stgs-eml/
- Sulfamethoxazole-Trimethoprim. (2017). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. https://www.ncbi.nlm.nih.gov/books/NBK547937/
Evaluation – Questions & answers
What is the most likely cause of the symptoms this patient is suffering from?
What are the side-effects and toxicities of ARVs within the first few weeks of starting therapy?
- Gastrointestinal toxicities include nausea, vomiting and diarrhoea. These side-effects are usually self-limiting and require symptomatic treatment only.
- Rash and liver toxicity are more common with the NNRTI drugs but are also seen with certain NRTI drugs such as abacavir (ABC) and some protease inhibitors (PIs).
- A lead-in dose is used for nevirapine (NVP) to lower the risk of toxicity.
- In case of mild-to-moderate rash and liver toxicity, antiretrovirals (ARVs) can be continued under close follow up, and symptomatic treatment and supportive care given.
- Severe rash and liver toxicity (ALT >5 ULN) can be life-threatening and NVP should be substituted with another drug.
- CNS toxicity from Efavirenz (EFV) can be self-limiting. Because EFV can cause dizziness most physicians advise that it should be taken at night.
- ABC hypersensitivity usually occurs within the first 6 weeks and can be life-threatening. ABC must be stopped and re-challenge never attempted.
What are the side-effects and toxicities of ARVs after 4 weeks of starting therapy?
- Drug-induced bone-marrow suppression such as anaemia and neutropenia are most commonly seen with AZT.
- Other causes of anaemia should be looked for and treated.
- Asymptomatic mild anaemia is common.
- If there is severe anaemia (Hb
What are the side-effects and toxicities of ARVs from 6-18 months after starting therapy?
- Mitochondrial dysfunction is primarily seen with the NRTI drugs; these include lactic acidosis, hepatic toxicity, pancreatitis, peripheral neuropathy, lipoatrophy and myopathy.
- Lipodystrophy is frequently associated with d4T use and can cause permanent disfigurement.
- Lactic acidosis is rare and can occur at any time. It is particularly associated with d4T use. Severe lactic acidosis can be life-threatening.
- Metabolic disorders are more common with PIs and include hyperlipidaemia, fat accumulation, insulin resistance, diabetes and osteopenia.
- Stop the NRTI and switch to another drug with a different toxicity profile
What are the side-effects and toxicities of ARVs after a year of starting therapy?
- Nephrolithiasis is commonly seen with indinavir (IDV).
- Renal tubular dysfunction is associated with tenofovir disoproxil fumarate (TDF).
- Stop the PI and switch to another drug with a different toxity profile.
How is ARV toxicity severity managed?
Mild reactions. These are bothersome but they do not require a change in therapy
Grade 2 (moderate)
Moderate reactions. Some moderate reactions (e.g. lipodystrophy or peripheral neuropathy) require substitution. For other reactions continue ART as long as is feasible; if the patient does not improve on symptomatic therapy, consider single drug substitution.
Grade 3 (severe)
Severe reactions. Substitute the offending drug without discontinuing ART.
Grade 4 (life threatening)
Severe life-threatening reactions. Immediately discontinue all ARV drugs and manage the medical event (i.e. symptomatic and supportive therapy) and reintroduce ARVs using a modified regimen (i.e. substituting another ARV drug for the offending drug) when the patient’s condition is stable. Hospitalization required.
Published by International Center for AIDS care and treatment programs- School of Public Health, Columbia University.
What are the indications for co-trimoxazole therapy?
What are the side effects of co-trimoxazole?
Co-trimoxazole is composed of two parts, trimethoprim and sulfamethoxazole (to find out more). Side effects of the trimethoprim component occur more frequently then side effects to sulfamethoxazole. The effects of trimethoprim are dose dependent, usually not severe, rarely of clinical significance and easily treated or prevented by folate supplementation. In contrast the side effects of sulfamethoxazole occur less often but are more severe. They are similar to the hematological side effects of other sulfonamide drugs which include hepatotoxicity, skin rash, blood dyscrasias, nephritis and cardiotoxicity. They are unpredictable and mostly mediated by the immune-system. They may be of life-threatening severity.
How do you manage co-trimoxazole toxicity?
What is the mechanism of action of co-trimoxazole toxicity?
The effects of these altered function is that patients who are slow acetylators develop toxicity because more drug is available for metabolism. The other effect is the production of hydroxylamine from cytochrome P-450 mixed-function oxidases. This metabolite is partly detoxified by conjugation with glutathione however people who develop toxicity to cot-trimoxazole have lymphocytes with glutathione synthetase deficiency.
In addition to chemical reactivity to these by products there can be a cell mediated immune response involving protein conjugation to the chemical by products.