- Patient presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Special investigations
- Discussion
- Final Outcome
- References
- MCQs
Patient presentation
Patient is a 22 year old female who presented to the surgery department of a tertiary level hospital having been referred from a private clinic, with a two month history of severe abdominal cramps, persistent bloody and mucoid diarrhoea, weight loss and tiredness.
Acknowledgement
This case study was kindly provided by Dr Monica Mercer from Immunopaedia
History
2 months ago:
Symptoms began with abdominal cramps and an intense urge to pass stool after every meal. Her symptoms rapidly worsened with passage of stool becoming more frequent. Within two days she was passing persistently watery diarrhoea mixed with fresh blood and mucous. She was seen by her general practitioner who treated her for gastritis.
One week later she collapsed at home and was admitted to hospital for investigations. She was discharged two days later without a diagnosis.
1 month ago:
Symptoms persisted and she experienced diarrhoea and vomiting after eating or drinking, which lasted for 10 days. She was admitted to hospital for rehydration and further investigations. No conclusive diagnosis was made.
Currently:
Patient is passing 10-20 liquid stools per day. Diarrhoea is mucoid and bloody. Occurs day and night. Patient complains of malaise, lethargy and anorexia. She has lost 8 kg in the past 2 months.
No past surgical history
No significant medical history
Family history:
Mother – type 2 Diabetes Mellitus
No other family members with chronic disease
No known allergies
Differential Diagnosis
- Infection:
- Cryptosporidium,
- Shigella,
- salmonella,
- E.coli,
- Campylobacter,
- Clostridium difficile
- If HIV positive consider- MAC, Isospera beli, cryptosporidium, TB
- Functional bowel syndromes e.g. irritable bowel syndrome (IBS)
- Malabsorbtion
- Coeliac disease
- Inflammatory bowel disease (IBD)
Examination
Thin ill looking young woman, conscious and alert, in obvious discomfort.
Vitals:
Heart rate: 80bpm
Respiratory rate: 18 bpm
Blood pressure: 120/70
Temperature: 37˚C
Pale mucous membranes
Abdominal examination:
Guarding and tenderness noted in the left iliac fossa and hypogastrium.
Investigations
No results available from previous admissions. All results are from current admission.
| FBC: | ||
|---|---|---|
| WCC | 5.9 x 10ˆ9/l | (4.00 – 10.00) |
| Hb | 9.0 g/dl | (12.1 – 15.1 g/dl) |
| Platelets | 748 x 10ˆ9/l | (150 – 400) |
| CRP | 17.4 mmol/l | (0 – 10 mmol/l) |
| Urea and Electrolytes: | ||
| Na | 137 mmol/l | (135 – 147 mmol/l) |
| K | 3.5 mmol/l | (3.3 – 5.0 mmol/l) |
| Cl | 96 mmol/l | (99 – 113 mmol/l) |
| Co2 | 31 mmol/l | (18 – 29 mmol/l) |
| Urea | 3.3 mmol/l | (2.5 – 7.0 mmol/l) |
| Creat | 32 umol/l | (60 – 12 umol/l) |
| Liver Function Test: | ||
| Total Bili | 7 umol/l | (3 – 18 umol/l) |
| Conjugated Bili | 3 umol/l | (0 – 5 umol/l) |
| Total Protein | 56 g/l | (60 – 80 g/l) |
| ALP | 66 U/l | (30 – 120 U/l) |
| GGT | 15 U/l | (5 – 35 U/l) |
| AST | 23 U/l | (5 – 45 U/l) |
| ALT | 18 U/l | (5 – 45 U/l) |
| Thyroid Function Test: | ||
| Free T4 | 15.1 pmol/l | (10.3 – 21 pmol/l) |
| TSH | 1.8 mIU/l | (0.35 – 4.50 mIU/l) |
| Calcium | 2.23 mmol/l | (2.12 – 2.65 mmol/l) |
| Albumin | 27 g/l | |
| Phosphate | 0.9 mmol/l | (0.8 – 1.4 mmol/l) |
| HIV ELISA | Negative | |
| Urine MCS: | ||
| Leucocytes | Trace | |
| Bacteria | Absent | |
| Stool MCS: | ||
| Brown | Unformed | |
| PMN | +++ | |
| Erythrocytes | ++ | |
| Parasites | Not observed | |
| Aerobic organisms | Not isolated | |
| C difficile toxin A | Negative | |
| C difficile toxin | Negative |
Special investigations
Abdominal X-ray:
No toxic megacolon
Gastroscopy Report:
Oesophagus and gastro- oesopahageal junction were normal. Stomach mucosa was intact and normal. No gastritis, ulceration or blood was noted. Cardia was normal. Pylorus and duodenum normal.
Colonoscopy report:
Very friable mucosa. Extensive ulceration with pseudopolyps, involving the rectum, entire sigmoid and left colon up to the transverse colon. Multiple biopsies of the colonic tissue were taken for histological analysis.
Histological Findings:
Pathology is limited to the mucosa and submucosa. Intense infiltration of the mucosa and submucosa with neutrophils and crypt abscesses, lamina propria with lymphoid aggregates, plasma cells, mast cells and eosinophils, and shortening and branching of the crypts.
Discussion
What is the Diagnosis?
Ulcerative Colitis, which is a chronic disease associated with diffuse mucosal inflammation of the colon, giving rise to significant morbidity and recurrent symptoms of intermittent bloody diarrhea, rectal urgency and tenesmus. Patients also present with fever, anemia, fatigue, weight loss, loss of appetite, loss of body fluids and nutrients, skin lesions, joint pain, and failure to grow. The latter is specifically seen in children. About half of the people diagnosed with ulcerative colitis have mild symptoms (Ulcerative colitis, no date). Onset of symptoms typically occurs between 15 and 40 years of age, with a second peak in incidence between 50 and 80 years of age.
Ulcerative colitis is closely related to another inflammatory intestinal condition called Crohn’s disease, which can lead to chronic inflammation in any part of the gastrointestinal tract. Together, these two conditions are collectively referred to as inflammatory bowel disease, or IBD. (Ulcerative colitis, no date).
Men and women are equally likely to develop ulcerative colitis. Extraintestinal manifestations may occur in up to 25% of patients. These include osteoporosis in 15%, oral ulcerations in 10%, arthritis in 5% to 10%, primary sclerosing cholangitis in 3%, uveitis in 0.5% to 3%, pyoderma gangrenosum in 0.5% to 2.0%, deep venous thrombosis in 0.3% and pulmonary embolism in 0.2%. Current cigarette smoking is associated with a reduction in the risk for ulcerative colitis, but former smokers have a higher risk of developing ulcerative colitis vs never smokers. Although the exact cause of ulcerative colitis is still unknown, there is strong evidence that primary dysregulation of the mucosal immune system causes an excessive immunologic response to normal microflora. Other contributing factors to ulcerative colitis are taken to be both genetic and environmental in nature (Richards, 2019).
What is the pathogenesis of ulcerative colitis?
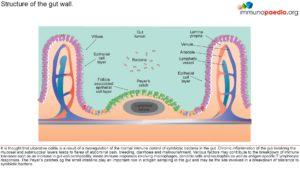 The gastrointestinal innate and adaptive immune system continuously faces the challenge of potent stimuli from the commensal microflora and food. These local immune responses require a tight control, the outcome of which is in most cases the induction of tolerance. Local T cell immunity is an important compartment of the specific intestinal immune system. T cell reactivity is programmed during the initial stage of its activation by professional presenting cells. Mucosal dendritic cells (DCs) play a key role in regulating immune responses in the antigen-rich gastrointestinal environment. Mucosal DCs are a heterogeneous population that can either initiate immune responses, or control intestinal inflammation and maintain tolerance. This breakdown of immune tolerance leads to the two major forms of inflammatory bowel disease, Crohn’s disease and ulcerative colitis (See figures 1 & 2). With IBD, what we know very well is that there is a skewed presence of T-cells, with more Th1 cells and pro-inflammatory cytokines (Richards, 2019) .
The gastrointestinal innate and adaptive immune system continuously faces the challenge of potent stimuli from the commensal microflora and food. These local immune responses require a tight control, the outcome of which is in most cases the induction of tolerance. Local T cell immunity is an important compartment of the specific intestinal immune system. T cell reactivity is programmed during the initial stage of its activation by professional presenting cells. Mucosal dendritic cells (DCs) play a key role in regulating immune responses in the antigen-rich gastrointestinal environment. Mucosal DCs are a heterogeneous population that can either initiate immune responses, or control intestinal inflammation and maintain tolerance. This breakdown of immune tolerance leads to the two major forms of inflammatory bowel disease, Crohn’s disease and ulcerative colitis (See figures 1 & 2). With IBD, what we know very well is that there is a skewed presence of T-cells, with more Th1 cells and pro-inflammatory cytokines (Richards, 2019) .
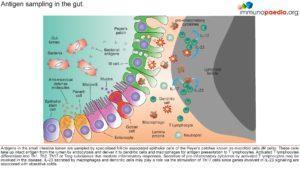 Microfold (M) cells are specialised epithelial cells of the Peyer’s patches that sample antigens from the gut lumen, thereby enabling the host to respond immunologically. These cells take up intact antigen from the lumen by endocytosis and deliver it to dendritic cells and macrophages for antigen presentation to T lymphocytes. Activated T lymphocytes differentiate into Th1, Th2, Th17 or Treg subclasses that mediate inflammatory responses. With IBD, what we know very well is that there is a skewed presence of T-cells, with more Th1 cells and pro-inflammatory cytokines present. This in turn leads to an increased presence of CD8 T-cells in the epithelial lining of the colon of UC patients , which is taken to contribute to damage of the lumen of the colon (See figure 2). UC can therefore be classified as a type 4 hypersensitivity reaction characterised by a heavy involvement of T-cells. With the increased presence of pro-inflammatory cytokines, B-cells are soon to follow and be involved too (Richards, 2019).
Microfold (M) cells are specialised epithelial cells of the Peyer’s patches that sample antigens from the gut lumen, thereby enabling the host to respond immunologically. These cells take up intact antigen from the lumen by endocytosis and deliver it to dendritic cells and macrophages for antigen presentation to T lymphocytes. Activated T lymphocytes differentiate into Th1, Th2, Th17 or Treg subclasses that mediate inflammatory responses. With IBD, what we know very well is that there is a skewed presence of T-cells, with more Th1 cells and pro-inflammatory cytokines present. This in turn leads to an increased presence of CD8 T-cells in the epithelial lining of the colon of UC patients , which is taken to contribute to damage of the lumen of the colon (See figure 2). UC can therefore be classified as a type 4 hypersensitivity reaction characterised by a heavy involvement of T-cells. With the increased presence of pro-inflammatory cytokines, B-cells are soon to follow and be involved too (Richards, 2019).
What gene associations occur in ulcerative colitis?
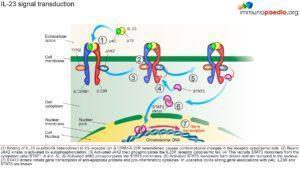 Gene association studies in patients with ulcerative colitis have identified the potential involvement of p40, IL-23R and STAT3 genes. These genes are involved in the signal transduction pathway of IL-23. IL-23 is a cytokine produced by macrophages and dendritic cells in immune responses to microbial pathogens. IL-23 stimulates the expansion of CD4+ helper T lymphocytes that have differentiated into Th17 phenotypes. Th17 T lymphocytes produce IL17 and other pro-inflammatory cytokines. Stimulation of the IL-23 receptor specifically leads to the transcription of genes encoding anti-apoptotic proteins and pro-inflammatory cytokines via the activation of STAT nuclear transcription factors. It is thought that the induction of a pro-inflammatory state of the immune system in response to normal gut bacteria causes the symptoms of ulcerative colitis. A strong association of the genes encoding p40 which is a subunit of IL-23 cytokine, IL-23R which is a subunit of the IL-23 receptor and STAT3 which is a nuclear transcription factor has been established in patients with ulcerative colitis. This suggests the involvement of Th17 helper T lymphocytes in the inflammatory response to gut bacteria in ulcerative colitis (See figure 3).
Gene association studies in patients with ulcerative colitis have identified the potential involvement of p40, IL-23R and STAT3 genes. These genes are involved in the signal transduction pathway of IL-23. IL-23 is a cytokine produced by macrophages and dendritic cells in immune responses to microbial pathogens. IL-23 stimulates the expansion of CD4+ helper T lymphocytes that have differentiated into Th17 phenotypes. Th17 T lymphocytes produce IL17 and other pro-inflammatory cytokines. Stimulation of the IL-23 receptor specifically leads to the transcription of genes encoding anti-apoptotic proteins and pro-inflammatory cytokines via the activation of STAT nuclear transcription factors. It is thought that the induction of a pro-inflammatory state of the immune system in response to normal gut bacteria causes the symptoms of ulcerative colitis. A strong association of the genes encoding p40 which is a subunit of IL-23 cytokine, IL-23R which is a subunit of the IL-23 receptor and STAT3 which is a nuclear transcription factor has been established in patients with ulcerative colitis. This suggests the involvement of Th17 helper T lymphocytes in the inflammatory response to gut bacteria in ulcerative colitis (See figure 3).
The array of genetic polymorphisms associated with UC would point to the likelihood of abnormalities in the epithelial barrier contributing to the onset of this condition, one hypothesis supporting the presence of an epithelial cell defect that initiates the disease under pressure from the colonic microbiome (figure 4) (Fuss and Strober, 2015).
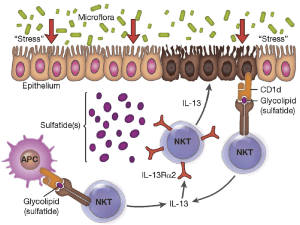
Figure 4:” Proposed mechanism of immune-mediated inflammation in UC. Inflammation in UC is initiated by release of glycolipid antigen(s) arising from genetically impaired epithelial cells under stress from exposure to components of the gut microbiome. These antigens are presented to and stimulate NK T cells in the context of CD1 on the surface of epithelial cells or on lamina propria dendritic cells. The NK T cells so stimulated cause epithelial cell damage by direct cytotoxic activity via interaction with CD1d loaded with glycolipid on the epithelial cell surface. Alternatively, the NK T cells cause epithelial apoptosis by release of IL-13 that then causes epithelial damage. Interleukin-13 also enhances inflammation by interacting with IL-13Rα2 on NK T cells, thereby inducing further NK T cell cytotoxic activity. Finally, epithelial ulceration resulting from these processes allows entry of bacterial components into the lamina propria that stimulates secondary inflammatory reactions.” Source: (Fuss and Strober, 2015)
What are Peyer’s patches?
Peyer’s patches are aggregations of lymphoid tissue, made up of lymphoid follicles located in the lamina propria of the mucosa. In adults, B lymphocytes are seen to predominate in the follicles’ germinal centers. T lymphocytes are found in the zones between follicles. With the lumen exposed to to the external environment, there are large numbers of potentially pathogenic microorganisms present. Peyer’s patches therefore carry out immune surveillance containing macrophages, dendritic cells, B-lymphocytes, and T-lymphocytes. The lymphoid tissue is covered by a special epithelium that contains specialized cells called M cells which sample antigen directly from the lumen and deliver it to antigen-presenting cells. These cells then pass to the mesenteric lymph nodes where the immune response is amplified.
How do you grade the severity of the disease?
Ulcerative Colits disease severity (based on Truelove and Witt classification):
- Symptoms Mild Severe Fulminant
- Stools per day 6 >10
- Hematochaezia Intermittent Frequent Continuous
- Temperature Normal >37.5 C
- Pulse Normal >90
- Haemoglobin Normal <75% of normal Transfusion
- ESR 30mm/hr
Download images for case
Final Outcome
Treatment and management:
On admission patient was rehydrated and given Solucortef 100 mg IMI tds. She continued to pass 10 stools the following day.
Day 3:
Patient continued to experience diarrhoea and unable to tolerate food or water.
Transfused with 2 units of packed cells
Prescribed:
Asacol 1.2g po, tds (mesalazine)
Asacol suppository PR bds
Morphine 15mg IMI PRN
Flagyl 500mg tds
Day 6:
Patient has continued to experience diarrhoea of watery, bloody stools. Abdominal pain has decreased and abdomen is soft and undistended.
It was decided to continue medical management for a further 7 days, with the addition of:
Cyclosporine 80mg IVI, infused over 2hrs
Losec 20 mg po daily (omeprazol)
Slow K rider IVI bds
Slow Magnesium IVI daily
Clexane 40 mg S/C daily (enoxaparin)
Day 13:
It was decided that medical management had failed as no relief of symptoms was achieved. Surgical management was therefore required.
A laparoscopic total colectomy and ileostomy was performed. Three months post surgery the patient is scheduled to return for ileal-anal pouch surgery, to eliminate the need to wear a bag.
Download images for case
- Ulcerative colitis (no date) MedicineNet. Available at: https://www.medicinenet.com/ulcerative_colitis/article.htm (Accessed: November 14, 2022).
- Richards, M. (2019) Immunopathogenesis of Ulcerative Colitis. USA: Maureen Richards Immunology & Microbiology: YouTube channel. Available at: https://www.youtube.com/watch?v=FnahtfSmP60.
- Fuss, I. J. and Strober, W. (2015) “Ulcerative Colitis,” in Mucosal Immunology. Elsevier, pp. 1573–1612.
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for case










