- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & answers
Patient Presentation
A 10 year old girl recently diagnosed with HIV (WHO stage 4), presents with a 12 day history of diarrhoea, vomiting, difficulty swallowing and abdominal cramps. She has remained afebrile throughout her illness.
Acknowledgement
This case study was kindly provided by Dr Bhadrish J Mistry, Paediatrician, Department of Paediatrics, Chris Hani Baragwanath Hospital and the University of Witwatersrand.
History
Six months ago she had been admitted to her local hospital following a 5 day history of swollen painful gums with painful swelling of the left upper jaw and cheek. She was diagnosed with acute necrotising ulcerative gingivitis (cancrum oris).
A month later she was started on anti-TB treatment based on scanty AFB found in her sputum, but she soon defaulted on her treatment.
2 weeks ago she was diagnosed with HIV (WHO 4). She was found to be severely wasted, with a CD4 count of 0,48% (4cells/ul) and a viral load of 140 000 copies/ml.
Differential Diagnosis
- Chronic gastroenteritis
- MDR TB
- Hepatitis
- Mycobacterium Avium Complex
- CMV
- EBV
Examination
General
- Cachectic (weight 44% expected weight for age) and stunted
- Well hydrated
- Not jaundiced or pale
- Generalised significant lymphadenopathy
- No petechia or purpura
Oral
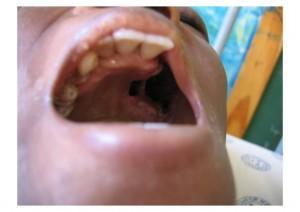
- Extensive oral candidiasis, with limited opening of the mouth
- Hard palate ulcerative lesion extending from the alveolus to the palatine process of the maxilla to the palatoglossal arch
- The defect has a rounded edge and a small posterior area with exudates
Abdomen
- Scaphoid, non tender
- 3-4 cm firm hepatomegaly but no splenomegaly
- No ascites
Respiratory
- Not in respiratory distress
- No features of chronic lung disease
Cardiovascular
- No abnormalities detected
- No features of cor pulmonale
Neurological
No focal signs
Dermatological
No rashes or kaposi lesions
Investigations
| Urine Dipstick | NAD |
|---|---|
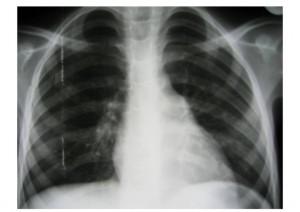
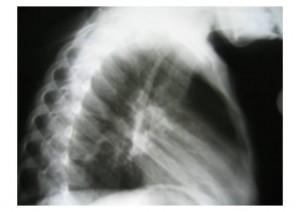
| Chest X-Ray | PA and Lateral |
| FBC: | |
| WCC | 4.5999999999999996 |
| Hb | 8.1999999999999993 |
| MCV | 103 |
| Plt | 357 |
| Differential: | |
| Neutrophils | 85 |
| Monocytes | 5 |
| Lymphocytes | 9 |
| CRP: | 58 |
| U/E: | |
| Sodium | 136 |
| Potassium | 2.5 |
| Chloride | 102 |
| C02 | 17 |
| Urea | 3.5 |
| Creatinine | 37 |
| Iron Studies: | |
| Fe | 5.2 |
| TF | 1.1000000000000001 |
| % Sat | 14 |
| Ferritine | 544 |
Hepatitis Studies: Negative
CMV (PP65): Negative
EBV: Negative
Abdominal Ultrasound
Liver : R lobe 13.5cm, L lobe 12.7cm, echogenic but no focal lesions or dilated ducts
Normal gallbladder, pancreas & spleen
Normal kidneys
No free fluid
Mesenteric & para-aortic nodes noted
Liver Biopsy
Not done
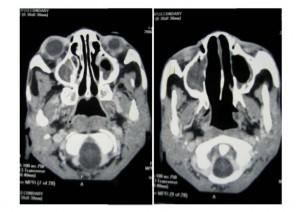
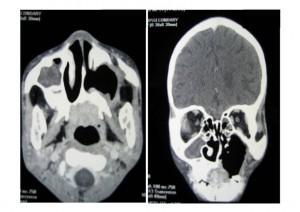
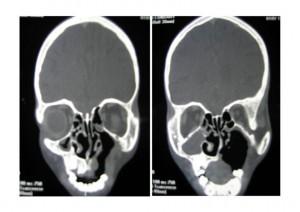
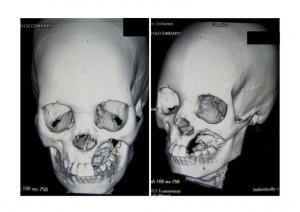
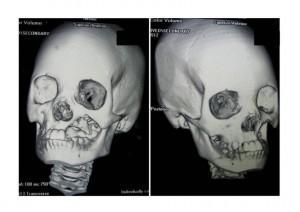
CT Scan of Face and Brain
Shows destruction of the left maxilla
Biopsy of the Hard Palate
Features of acute on chronic inflammation
No evidence of granulomas
Culture of Hard Palate Biopsy
Isolated Mycobacterium Avium Complex and Candida Krusei
3 Sputum Specimens
Ziehl-Neelsen Stain – Numerous AFBs
Culture isolated Mycobacterium Avium Complex (MAC)
2 TB Bactec Specimens
Isolated AFB positive – MAC
Bone Marrow Aspirate & Trephine
Showed active haematopoeisis
Ill-defined granuloma showing epitheliod histiocytes
Decreased megakaryocytes, granulopoeisis & erythropoeisis
Discussion
There are many causes of oral ulcerative lesions. Listed here are the ones that are most commonly encountered:
- Lymphoma
- Necrotising Ulcerative Gingivitis
- Necrotizing Ulcerative Peridontitis – associated with severe pain and bleeding, rapid loss of bone and soft tissue
- Necrotising Stomatitis
- Mycobacterium Avium Complex (MAC) this is quite rare
- The herpes group of infections
- CMV – Necrotic with a white halo
- Fungal infections including Histoplasmosis and Cryptococcosis
- Aphthous ulcers
- Neutropenic ulcerationis
- Acute Necrotising Gingivitis (risk factors include poverty, malnutrition, poor oral hygiene and infectious diseases)
MAC oral manifestation
There is not a lot of information regarding the oral manifestation of MAC however, two reported adult cases have shown it to first present as a palatal and gingival ulcerative lesion with firm borders and a necrotic centre that extended down to the bone and later invading the maxillary, frontal, sphenoid and ethmoid sinuses. MAC was isolated from sputum, blood, bone marrow and the lesion itself in both patients.
Epidemiology of MAC
This is a multiple related species of nontuberculous mycobacteria including M avium, M. intracellulare and M. paratuberculosis. It is the second most common opportunistic infection found in HIV infected children.
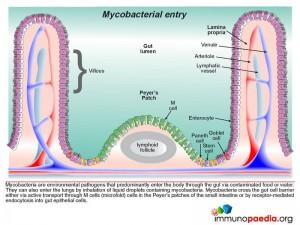
It is a ubiquitous pathogen found throughout the environment often in contaminated water and food, transmitted via ingestion, inhalation or inoculation, leading to GIT and pulmonary disease. Although disseminated infection rarely occurs during the first year of life it occurs more frequently thereafter.
Risk factors for MAC
- CD4 count 50 cells/ul (and in children can occur at higher CD4 counts)
- High viral load 100 000 RNA copies/ml
- Prior opportunistic infections (esp CMV disease)
Pathophysiology
- The disease is not due to reactivation but rather recent acquisition.
- Typically the host reaction is minimal with little occurrence of tissue destruction.
- The organism adheres to the gut wall with rapid penetration of the intestinal mucosa.
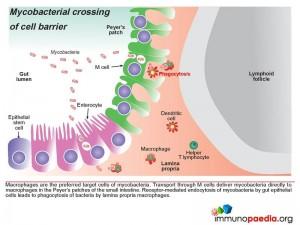
- Macrophages are the preferred target cells of mycobacteria. The bacteria are transported through M cells directly to macrophages in the Peyer’s patches of the small intestine. Where receptor-mediated endocytosis of mycobacteria by gut epithelial cells leads to phagocytosis of bacteria by lamina propria macrophages.
- Phagocytosis by macrophages in advanced AIDS is usually impaired.
- Phagocytosis of Mycobacteria by macrophages internalises bacteria in a membrane-bound phagosome. Under normal conditions, the phagosome fuses with a lysosome forming a phagolysosome which kills the bacteria. Mycobacterium tuberculosis and Mycobacterium avium have evolved mechanisms to prevent the fusion of the lysosome with the phagosome thus evading host immune defenses by allowing the bacteria to survive and multiply in the phagosome.
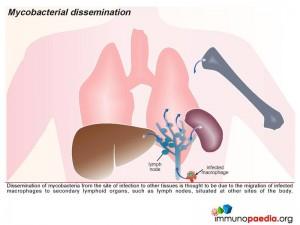
In this way they can spread to other parts of the body when the macrophages migrate to other tissues.
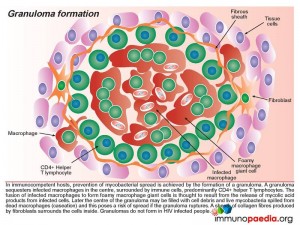
- In immunocompetent hosts, prevention of mycobacterial spread is achieved by the which sequesters infected macrophages in the centre, surrounded by predominantly CD4+ helper T lymphoctes. The infected macrophages fuse and form giant cells. Later the centre of the granuloma may be filled with cell debris and live mycobacteria spilled from dead macrophages which poses a risk of spread if the granuloma ruptures. A sheath of collagen fibres produced by fibroblasts surrounds the cells.
- Granulomas do not form in HIV infected peoples.
- Factors which contribute to growth of MAC
- High levels of triglycerides.
- Iron overload.
- Factors contributing to killing defect in advanced AIDS
- Absence of cytokine production.
- Impaired cell-mediated immunity.
- Impaired granuloma formation
Disseminated MAC
- All areas of the GIT are involved, most commonly the duodenum; the disease is usually patchy.
- If there is unimpeded replication massive thickening of the gut wall occurs. Which is the most likely reason for the abdominal pain found in this patient.
- Spread occurs to lymph nodes via local lymphatics.
- Bacterial replication occurs in lymph nodes and subsequent rupture may occur which results in haematogenous spread.
- Bacteria are taken up by phagocytic cells throughout the body and the reticuloendothelial system (liver, spleen & bone marrow).
- Genital ulcers
- CNS infection
In the absence of ARVs
- Disseminated multi-organ infection (early symptoms can be minimal).
- Features are:
- Fever, night sweats, cachexia (major symptoms)
- Diahorrea, nausea
- Abdominal pain
- Intra-abdominal lymphadenopathy & hepatosplenomegaly
- Anaemia (most likely anaemia of chronic disease).
- Markedly elevated ALP with little elevation of transaminases or bilirubin.
- Other manifestations – sinusitis, oral manifestations.
- Respiratory symptoms are uncommon in children with dissemination and isolated pulmonary disease is rare.
Diagnosis
- Is made from isolation of the organism from blood specimens using Bactec or biopsy specimens of normally sterile sites.
- Multiple mycobacterial blood cultures may be required over a time frame due to fluctuating levels of bacteraemia.
- MAC in stool or sputum indicates colonization but not necessarily invasive disease.
- Histological features include:
- Granulomas (necrotising & non-necrotising).
- Sheets of macrophages laden with AFB.
- Necrotising acute & chronic inflammation with histiocytes.
- Massive histiocyte infiltrate with intracellular AFB.
Conclusion
- MAC represents a late stage event in AIDS-related disease.
- Presentation may be localised or involve multiple organs.
- Disseminated disease remains a substantial cause of morbidity & mortality.
- ARV’s & combination therapy- improve survival.
- Oral manifesations are rare.
- In this patient the oral lesion may be the primary manifestation of disseminated disease.
- Enlarged liver and abdominal pain may also be due to dissemination, biopsies will need to be done to confirm this.
Download images for this case
Treatment
The patient was commenced on clarithromycin, ethambutol and ciprofloxacin.
After 5 weeks of MAC treatment ARV’s were commenced; stavudine (d4T), lamivudine (3TC), efavirenz (Stocrin).
In view of the drug interactions known to occur between efavirenz and clarithromycin (effect of P450 metabolism) and the subsequent inadequate treatment of the MAC infection (positive MAC on TB Bactec after 2 months of treatment) azithromycin was commenced.
Download images for this case
Final Outcome
- Gradually the child’s appetite and weight improved.
- Viral load was suppressed
- CD4 count had increased to 1.08% (16cells/ul).
- A liver function test showed elevations in ALP, GGT, AST and ALT. This may be due to disseminated MAC however no liver biopsy was taken to confirm this.
- Hepatitis studies were negative.
- No further outcomes are yet available on this patient.
Download images for this case
References
Greenspan D et al. (1997). Oral manifestations of HIV infection. Aids Clinical Care. 9:29-33.
Link to Abstract
Novak MJ. (1999) Necrotizing Ulcerative Peridontitis. Annals of Peridontology. 4:74-77.
Link to Abstract
Mayer DM. (2003) Noma: an “infectious” disease of unknown aetiology. The Lancet. 3:419-431.
Link to Abstract
Robinson P et al (1996). Oral Mycobacterium avium complex infection in a patient with HIV-related disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 81:177-179.
Link to Abstract
Volpe DDS et al. (1985) Oral manifestation of disseminated Mycobacterium avium intracellulare in a patient with AIDS. Oral Surgery. 60:567-570.
Link to Abstract
CDC. (2004) Mycobacterium avium Complex Disease. MMWR. 53:1-63.
Link to Abstract
Horsburgh CR. The pathophysiology of Disseminated Mycobacterium avium Complex Disease in AIDS. The Journal of Infectious Diseases. 1999;179(Suppl 3):S461-5
Download images for this case
Evaluation – Questions & answers
What is the diagnosis
How does Mycobacterium Avium Complex predominantly enter the body?
Which cells are typically involved in mycobacterium infections?
How do mycobacterium survive and spread throughout the body?
How is mycobacterium typically contained to prevent it from infecting the host?
Will granulomas form in HIV infected patients?
Download images for this case