- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Case Treatment
- Final Outcome
- References
- Evaluation - Questions & answers
- MCQ
Patient Presentation
A 10 year old boy presents with a 3 week history of weight loss, diarrhoea, vomiting and a cough. No toxin ingestion has been noted and the family denies any use of traditional medicines.
Acknowledgement
This case study was kindly provided by Dr Bhadrish J Mistry, Paediatrician, Department of Paediatrics, Chris Hani Baragwanath Hospital and the University of Witwatersrand.
History
A 10 year old boy was brought into the emergency department by his mother because she was worried about ongoing diarrhoea for three weeks and vomiting following every meal. As a result he had lost a lot of weight and was extremely thin. She also noted that he was having difficulty walking and sitting up, and his hands would shake as he tried to pick things up. The mother was also worried about an on going cough. Although she had not noted any fever or night sweats and the cough was non-productive, she had been infected with TB one year before. She had completed her treatment and was now sputum negative, but the child had not received any TB prophylaxis at the time and she was worried he may also have TB.
Past Medical History
The mother reported occasional episodes of diarrhoea since age 1 year, which were treated at her local clinic. No other illnesses or hospitalisations were noted.
Birth History
The child was born term, with a normal vaginal delivery- no complications.
He was breast and formula fed, until age 2 years.
The mother does not know her HIV status, and was not treated with Nevirapine at the time of delivery.
Social History
The family live in Soweto in a two bedroom house. There are 4 adults and two older siblings aged 12 and 13, who are all reported as well. Two of the adults are employed and support the household. Water and electricity are available in the home.
Vaccinations
Up to date
Medication
No medication
No history of any traditional medication use (orally or via enema).
Allergies
None known
Differential Diagnosis
- Chronic gastroenteritis
- TB
- Extrapulmonary TB
- Cerebellitis (TB, viral, salmonella)
- HIV
Examination
Vitals
- Heart Rate- 130 beats per minute
- Blood Pressure- 76/52
- Respiratory rate- 22 breaths per minute
- Temperature- 38.0°C
Anthropometry
Severely wasted, weight 60% of expected weight for age (EWA)
General
- Mild pallor
- No jaundice
- No lymphadenopathy
- No oedema
- No dehydration
- No rashes
Cardiovascular
Normal heart sounds, no gallop, no murmurs
Chest
- Not in respiratory distress
- No adventitious sounds
Abdomen
- Not distended, non tender
- No organomegaly
- Normal bowel sounds present
Neurological
- Confused, waxing and waning level of consciousness
- No neck stiffness
- Fundoscopy, no abnormalities detected
- Cerebellar signs
- Truncal ataxia & mild gait ataxia
- No nystagmus
- Intention tremor & past pointing
- Dysdiadochokinesis
- Possible staccato speech
- Bilateral long tract signs
- Increased tone bilaterally, upper limbs and lower limbs
- Brisk reflexes globally but no plantar reflex
- Cross adductors
- No weakness
Intact sensation but positive Romberg sign
Investigations
| Values | Normal Limits | |
|---|---|---|
| WBC | 4.95 (diff 4.6 neutrophils and lymphocytes 0.3) | |
| HB | 9.6999999999999993 | (4-12x109/l) |
| MCV | 78.599999999999994 | (12.1-15.2 g/l) |
| Platelets | 195 | (140-450x109/l) |
| CRP | 110 | ( |
| ESR | 84 | |
| NA | 123 | (135-147 mmol/l) |
| K | 3.3 | (3.5-5.1 mmol/l) |
| CL | 84 | (95-107 mmol/l) |
| C02 | 23 | (22-23 mmol/l) |
| UREA | 7.2 | (2.5-6.7 mmol/l) |
| CREAT | 53 | (70-150 umol/l) |
| TBIL | 14 | (3-17 umol/l) |
| INDIR BIL | 1 | (0.0-0.3 mg/dl) |
| TPROT | 80 | (60-80 g/l) |
| ALB | 34 | (35-50 g/l) |
| ALP | 61 | (30-150 u/l) |
| GGT | 34 | (11-51 u/l) |
| ALT | 23 | (5-35 u/l) |
| AST | 193 | (5-35 u/l) |
Lumbar Punch
Protein: 0.25
Glucose: 3.5
Chloride: 112 No cells, culture negative
Blood Cultures:
Negative
Urine Culture:
Negative
PPD
Negative
CT Brain
Mild involutional changes, no space occupying lesions, no raised intracranial pressure.
Chest X-Ray
Hilar lymphadenopathy
No cavitations
The patient was treated empirically with high dose Cefoxatime.
Five days later
Patient still has a fever, he has now developed jaundice and abdominal distension.
Additional Investigations on day 5
Stool MC and S-negative
Widal – Negative
HIV Elisa – Reactive
| Values | Normal Limits | |
|---|---|---|
| WBC | 5.05 | |
| HB | --- | (4-12x109/l) |
| MCV | 77 | (80-96 fl)) |
| Platelets | 138 | (140-450x109/l) |
| TBIL | 22 | (3-17 umol/l) |
| INDIR BIL | 13 | (0.0-0.3 mg/dl) |
| TPROT | 66 | (60-80 g/l) |
| ALB | 27 | (35-50 g/l) |
| ALP | 111 | (30-150 u/l) |
| GGT | 65 | (11-51 u/l) |
| ALT | 26 | (5-35 u/l) |
| AST | 284 | (5-35 u/l) |
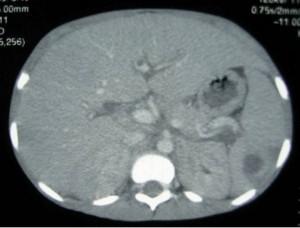
Abdominal Ultrasound
Hepatomegaly, coarse echogenic pattern, no focal lesions.
Splenomegaly, diffuse hypoechogenic lesions with large 2.8 x 1.6 cm hypoechogenic lesion.
Free fluid
Abdominal CT Scan
Hepatomegaly, no focal lesions.
Splenomegaly, 1.45 x 1.62 cm hypoattenuating lesion with no enhanced or thickened wall, no calcifications.
Moderate ascites.Para-aortic & coeliac axis lymph nodes.
Small right pleural effusion.
Bone marrow aspirate & trephine
Megaloblastoid features.
Disordered erythropoeisis.
Moderate-severe plasmacytosis – underlying chronic infection.
Ill defined granulomas
Discussion
Splenic tuberculosis although uncommon is considered an important manifestation of abdominal TB, especially in developing countries. Its prevalence is increasing with the epidemic of HIV-TB co-infection and subsequent rise in extrapulmonary TB. To best understand this disease process one has to look at the immunological processes which occur with dissemination of mycobacteria to extrapulmonary sites and HIV dissemination to secondary lymphoid tissue.
Dissemination of mycobacteria from the lung to other organs can occur when macrophages become infected with bacteria following phagocytosis Migration of activated macrophages to secondary lymphoid tissue for antigen presentation to CD4+ helper T lymphocytes can spread the bacteria to other tissues such as liver, lymph nodes, spleen, gut and bone marrow.
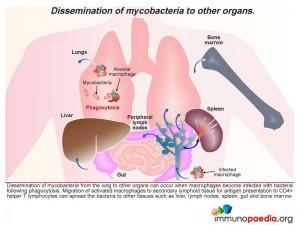 Looking specifically at the spleen which alongside removal of aged erythrocytes has a major function of detecting blood borne antigens entering the organ via arterial blood flow, we see inflammatory responses being initiated. This occurs in the lymphoid tissue known as the white pulp which is rich in lymphocytes and antigen-presenting cells. Foreign antigen is detected here and inflammatory responses become activated. The blood then continues to flow into specialised sinuses called the red pulp where macrophages remove aged erythrocytes by phagocytosis. Macrophages infected with mycobacteria may then enter the spleen and spread bacteria to other macrophages. In HIV infection the immune response to mycobacteria is weakened and bacteria are able to replicate more readily.
Looking specifically at the spleen which alongside removal of aged erythrocytes has a major function of detecting blood borne antigens entering the organ via arterial blood flow, we see inflammatory responses being initiated. This occurs in the lymphoid tissue known as the white pulp which is rich in lymphocytes and antigen-presenting cells. Foreign antigen is detected here and inflammatory responses become activated. The blood then continues to flow into specialised sinuses called the red pulp where macrophages remove aged erythrocytes by phagocytosis. Macrophages infected with mycobacteria may then enter the spleen and spread bacteria to other macrophages. In HIV infection the immune response to mycobacteria is weakened and bacteria are able to replicate more readily.
As explained TB infection occurs in macrophages which are found either in tissues or lymphoid organs. TB effector cells play a role in containing infection, which culminates in granulomatous formation. However in the presence of HIV these microenvironments become depleted of these antigen specific responses resulting in breakdown and no local TB control i.e. TB infection with no granulomatous formation.
Dissemination of HIV from the site of infection to secondary lymphoid tissue in the gut, spleen and peripheral lymph nodes occurs shortly after transmission. This can be due to migration of activated macrophages infected with HIV or migration of activated dendritic cells carrying surface-bound infectious virions to the paracortical zone of secondary lymphoid tissue for antigen presentation to T cells.
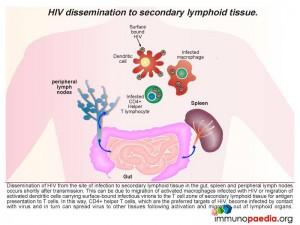 Macrophages infected with HIV can infect CD4+ T helper lymphocytes during cell contact in the T cell zone of lymphoid organs as well as dendritic cells carrying infectious virus particles on the cell surface. Follicular dendritic cells in the germinal centres of lymph nodes also trap infectious virus on the cell surface and can infect T cells entering the lymph node via afferent lymphatics or T cells associated with B cells in germinal centres. Activated CD8+ cytotoxic T cells expand in the lymph node to kill the infected T cells. Viral gp120 antigen accumulates in the lymph node and interacts with CD4 receptors on helper T lymphocytes causing retention of these cells. In this way, CD4+ T helper lymphocytes which are the preferred targets of HIV, become infected by contact with virus and in turn can spread virus to other tissues following activation and migration out of lymphoid organs.
Macrophages infected with HIV can infect CD4+ T helper lymphocytes during cell contact in the T cell zone of lymphoid organs as well as dendritic cells carrying infectious virus particles on the cell surface. Follicular dendritic cells in the germinal centres of lymph nodes also trap infectious virus on the cell surface and can infect T cells entering the lymph node via afferent lymphatics or T cells associated with B cells in germinal centres. Activated CD8+ cytotoxic T cells expand in the lymph node to kill the infected T cells. Viral gp120 antigen accumulates in the lymph node and interacts with CD4 receptors on helper T lymphocytes causing retention of these cells. In this way, CD4+ T helper lymphocytes which are the preferred targets of HIV, become infected by contact with virus and in turn can spread virus to other tissues following activation and migration out of lymphoid organs.
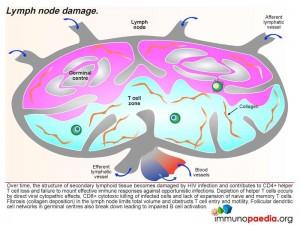
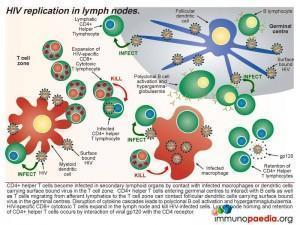 Over time, the structure of secondary lymphoid tissue becomes damaged by HIV infection and contributes to CD4+ helper T cell loss and failure to mount effective immune responses against opportunistic infections. Depletion of helper cells occurs by direct viral cytopathic effects, CD8+ cytotoxic killing of infected cells and lack of expansion of naive and memory T cells. The lymph node also becomes fibrosed due to collagen deposition which further impairs lymph node functioning. Clinically this is noted as small lymph nodes or no detectable lymphadenopathy.
Over time, the structure of secondary lymphoid tissue becomes damaged by HIV infection and contributes to CD4+ helper T cell loss and failure to mount effective immune responses against opportunistic infections. Depletion of helper cells occurs by direct viral cytopathic effects, CD8+ cytotoxic killing of infected cells and lack of expansion of naive and memory T cells. The lymph node also becomes fibrosed due to collagen deposition which further impairs lymph node functioning. Clinically this is noted as small lymph nodes or no detectable lymphadenopathy.
Download images for this case
Treatment
After 5 days the patient was commenced on metronidazole and cloxacillin. However he continued with persistent spiking fevers for a further two days.
The patient was then started on ciprofloxacin, amikacin and ethambutol.
Further investigations, day 10:
| Values | Normal Limits | |
|---|---|---|
| WBC | 2.7 | (4-12x109/l) |
| HB | 8.6999999999999993 | (12.1-15.2 g/l) |
| MCV | 77 | (80-96 fl)) |
| Platelets | 46 | (140-450x109/l) |
| TBIL | 59 | (3-17 umol/l) |
| INDIR BIL | 48 | (0.0-0.3 mg/dl) |
| TPROT | 55 | (60-80 g/l) |
| ALB | 22 | (35-50 g/l) |
| ALP | 104 | (30-150 u/l) |
| GGT | 91 | (11-51 u/l) |
| ALT | 73 | (5-35 u/l) |
| AST | 697 | (5-35 u/l) |
| INR | 1.83 | |
| D-DIMERS | 1.75 | |
| LDH | 4725 |
He then underwent a sonar guided aspiration of the splenic abscess, which yielded 10 ml of very viscous pus which was sent for MC&S and TB culture. The culture was positive for Mycobacterium tuberculosis.
Mycobacterium tuberculosis was also cultured in sputum and blood culture specimens.
The hepatitis resolved and the patient was commenced on 4 drug TB treatment.
Splenic TB is usually associated with dissemination, usually with accompanying liver involvement. There are 3 categories of tubercular abscesses of the spleen which include splenic, splenohepatic and splenohepatoglandular abscesses.
The gold standard for management of splenic abscess used to be splenectomy however splenic preservation has now been shown to be important because of the immunologic functions of the spleen, especially in children. Successful treatment of single and multiple splenic abscesses in children is therefore achieved with antibiotic therapy.
TB splenic abscesses in children is becoming increasing common since the advent of HIV.
Download images for this case
Final Outcome
- The patient’s weight has increased from 60 to 71% of expected weight for age.
- A repeated abdominal U/S showed no splenomegaly or focal lesions.
- The patient was then commenced on ARV’s. He was closely monitored for an IRIS reaction which did not occur.
- He has been improving and has been transferred to an outpatient paediatric HIV facility
Download images for this case
References
Lucien PJ et al. Splenic Abscesses from 1987 to 1995. The American Journal Of Surgery 1997;174:87-94
Link to Abstract
Liang JT. Splenic Abscess: A Diagnostic Pitfall in the ED. American Journal of Emergency Medicine 1995;13:337-43
Link to Abstract
Roy Choudhury S et al. Management of splenic abscess in children by percutaneous drainage. Journal of Pediatric Surgery 2006; 41: E53–56.
Link to Abstract
Estes J et al. The role of collagen doposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Seminars in Immunology 20(2008):181-186
Link to Abstract
Green D et al. Human Immunodeficiency virus type 1 gp120 reprogramming of CD4+ T-cell migration provides a mechanism for lymphadenopathy. Journal of Virology June 2009: 5765-5772
Link to Abstract
Santosuosso M et al. HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic hiv-1 infection. Journal of Infectious Diseases October 2009: 200
Link to Abstract
Lederman M and Margolis L. The lymph node in HIV pathogenesis. Seminars in Immunology. 20(2009): 187-195
Link to Abstract
Download images for this case
Evaluation – Questions & answers
What is the diagnosis?
Which cell is a target cell for mycobacteria in TB infections?
How does the bacteria spread to extrapulmonary tissues, including the spleen?
How does dissemination of HIV to secondary lymphoid tissues occur?
In the lymph node what happens to the infected T cells?
What happens to lymphoid tissue after years of HIV infection?
How does involuted lymphoid tissue manifest clinically?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case