- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- Evaluation - Questions & answers
Patient Presentation
A 7 year old girl presented to her local hospital for the second time in 3 months with arthralgia, a migratory skin rash and fever for 5 days. She was admitted and within 2 days developed a depressed level of consciousness, epistaxis and seizures.
Acknowledgement
This case study was kindly provided by Dr Monika Esser MMed Paed, Head of Division of Immunology, N.H.L.S Coastal Branch, Tygerberg Hospital.
History
Patient was diagnosed one year earlier with pulmonary TB (PTB) on positive cultures obtained from gastric washings. Her PTB infection was accompanied with symptoms of fever and torticollis. She was started on TB treatment but only completed 2 months of treatment.
Six months later she had not resumed TB treatment and was diagnosed with mitral regurgitation on echocardiography, with an elevated antistreptolysin O titre (ASOT) and an elevated anti-dnase test. She was admitted to hospital and treated for acute rheumatic fever (ARF) with benzyl penicillin. The mitral regurgitation normalised on echo and she was discharged.
Over the course of the following 3 months she developed progressive arthritis, progressive loss of joint function and a chronic remitting fever and rash.
Differential Diagnosis
- Post streptococcal arthritis
- Pulmonary TB
- Systemic Lupus Erythematosus
- Acute rheumatic fever
- Juvenile rheumatoid arthritis
Examination
An admission child appears miserable and toxic:
- Height and weight < 3rd centile
- Pyrexial – 38°C
- Pale
- Generalised lymphadenopathy
- Urticarial rash
- 2cm hepatosplenomegaly
- Tender and bilaterally limited range of motion in the wrists, knees, ankles and proximal and distal interphalangeal joints of the fingers
2 days later:
- Depressed level of consciousness
- Epistaxis
- Seizures
- Pyrexia ranging from 38-40°C
- Severe hepatosplenomegaly
- Consolidation of right upper lobe of the lung with bronchial breathing
 Discussion
Discussion
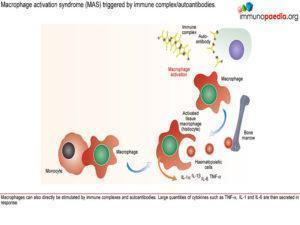
Based on the clinical presentation and investigations the differential diagnosis must now include Macrophage activation syndrome (MAS).
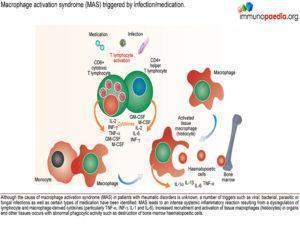
Cause:
- Unknown but a number of triggers such as infection (EBV, bacterial, parasitic and fungal) and certain types of medication have been identified.Excessive activation and proliferation of T lymphocytes and tissue macrophages (histiocytes) with massive cytokinaemia, including high levels of: TNF-a, INF-g, IL-1 and IL-6
- This produces an overwhelming, potentially fatal, inflammatory reaction. With increased recruitment and activation of tissue macrophages in organs and other tissues with abnormal phagocytic activity such as destruction of bone marrow haematopoeitic cells
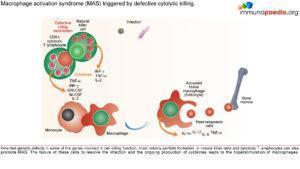
- Defective functioning of perforin (perforin is a protein involved in the cytolytic processes and control of lymphocyte proliferation).
- Non-remitting high fever
- Hepatosplenomegaly
- Lymphadenopathy
- Pancytopenia,
- Liver insufficiency,
- Coagulopathy
- Haemorrhages
- Neurological symptoms
- Cytopenia
- Abnormal liver function tests
- Coagulopathy
- Decreased erythrocyte sedimentation rate
- Hypertriglyceridaemia
- Hyponatremia
- Hypoalbuminemia
- Hyperferritinemia
- Histopathological features
- Macrophage haemo phagocytosis in the bone marrow
- Incidence is unknown
- Rare complication
- Most commonly affects children with systemic juvenile inflammatory arthritis (JIA) but has been observed in different subtypes of JIA or in other systemic diseases such as systemic lupus erythematosus (SLE)
- Generally develops in the earlier phases of the underlying disease and may occasionally be the presenting manifestation, but occurrence as late as 14 years after diagnosis has been reported
- In most patients, the primary disease is clinically active at the onset of MAS, but the syndrome may also develop during quiescent phases
- Triggered by flare-up of the underlying disease, aspirin or other nonsteroidal anti-inflammatory drug toxicity, viral infections, a second injection of gold salts, and sulfasalazine therapy.
Pathogenesis:
May be the result of a mutation in the perforin gene leading to decreased expression of perforin:
- Perforin – is a protein that is expressed in lymphocytes, macrophages, and other bone marrow precursors. Its main role in the cytolytic process is to form pores in the cell membrane, leading to osmotic lysis of the target cells.
- May also control lymphocyte proliferation
Diagnostic Guidelines:
Variables that showed the highest (0.75) sensitivity and specificity for MAS:
- elevated ferritin (above 10,000 ng/mL),
- elevated triglycerides (above 160 mg/dL),
- elevated aspartate aminotransferase (above 59 IU/mL),
- elevated fibrinogen (above 250 mg/dL),
- elevated alanine aminotransferase (above 40 IU/mL),
- elevated glutamyl transferase (above 40 IU/mL),
- low platelet count (below 262 x109/L),
- bone marrow aspirate showing hemophagocytosis
(b) Variables that did not prove sufficiently sensitive and specific:
- fever (38°C),
- lymphoadenopathy,
- neurologic manifestations
- arthritis,
- rash
- hemorrhages,
- decreased leukocyte count (below 4,00 x109/L),
- elevated erythrocyte sedimentation rate (above 50 mm/h),
- elevted lactate dehydrogenase (above 900 IU/mL)
- decreased serum sodium ( below 130 mEq/L).
Management: Treatment strategies for MAS
MAS is a potentially life threatening complication. It is therefore important to have a high threshold of suspicion in order to make an early diagnosis and start prompt treatment:
- Parenteral administration of high doses of corticosteroids.
- Cyclosporin A – treating severe or corticosteroid-resistant MA
- It causes a “switch-off” effect on the disease process, leading to resolution of fever and improvement of laboratory abnormalities
- Suppression of the early steps in T-cell activation
- Affects macrophage production of IL-6, IL-1, and TNF
- Inhibits the expression of inducible nitric oxide synthetase and cyclooxygenase-2 in macrophages.
- High-dose intravenous immunoglobulins
- Etanercept might be an effective adjunctive therapeutic agent in MAS.
Diagnostic Rule:
The diagnosis of MAS requires the presence of any 2 or more laboratory criteria or of any 2 or more clinical and/or laboratory criteria. A bone marrow aspirate for the demonstration of haemo phagocytosis may be required only in doubtful cases.
Laboratory criteria:
- Decreased platelet count (≤262 x 109/L)
- Elevated levels of AST (> 59 U/L)
- Decreased white blood cell count (≤ 4.0 x 109/L)
- Hypofibrinogenemia (≤2.5 g/L)
Clinical criteria:
- Central nervous system dysfunction (irritability, disorientation, lethargy, headache, seizures, coma)
- Haemorrhages (purpura, easy bruising, mucosal bleeding)
- Hepatomegaly (≥ 3cm below the costal arch)
Histopathological criterion:
Evidence of macrophage haemophagocytosis in the bone marrow aspirate
Download images for this case
Treatment
- The patient was treated with IVIG and repeated intravenous methylprednisolone pulsing.
Methotrexate was started with normalisation of liver functions. - She required repeat blood transfusions and was kept on broad spectrum antibiotic cover but all cultures remained negative.
Download images for this case
Final Outcome
- The patient proceeded to make a rapid and complete recovery and is currently maintained on low dose Methotrexate.
- A full course of TB treatment was completed with normalisation of liver functions.
Download images for this case
Evaluation – Questions & answers
What is the diagnosis?
What is the cause of Macrophage activation syndrome (MAS)?
What is the effect of increased levels of T-cell and macrophage derived cytokines, particularly TNF-a, INF-y, IL-1 and IL-6?
What is perforin?
What is the mechanism of macrophage activation syndrome (MAS)?
Download images for this case