- Patient presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final outcome
- References
- Evaluation - Questions & answers
- MCQ
Patient presentation
Four weeks after starting ARVs an 11 month old baby presented with a one-week history of a right axillary swelling which was found to be 2.5cm in diameter, hard, tender and non-fluctuant. Six days later the mass had enlarged to a size of approximately 4x3cm.
Acknowledgement
This case study was kindly provided by Dr Fatima Laher MBBCh, Dip HIV Man (SA) and Dr Gail Ashford MBBCh, DMH, Dip HIV Man (SA) from the Perinatal HIV research Unit, Chris Hani Baragwanath Hospital, Soweto. Additional input was also provided by Dr Lucy Connell, Harriet Shezi Clinic, Chris Hani Baragwaneth Hospital, Soweto.
History
The child was born an HIV-exposed infant and received a single-dose of nevirapine syrup, as well as the standard immunisations including the Bacille Calmette-Guérin (BCG) vaccine. The mother was counseled and requested to bring the child back for an HIV test at 6 weeks. However this was not done until 9 months later at which time the infant tested HIV positive on two rapid ELISA blood tests with a CD4 percentage of 9.28 (absolute count 378x10e6/L). Her caregivers then defaulted on a referral and telephonic reminders to initiate her on antiretroviral therapy.
One month later, the infant now 10 months old, presented with a two-week history of diarrhoea and a 4 month history of lethargy and failure to thrive. All immunisations were up to date and no TB contacts were identified. On examination she was found to be:
- Underweight (70% of expected weight for age)
- pale
- dehydrated
- shotty lymphadenopathy
- oral candidiasis
- bilateral otitis media
- hepatosplenomegally
- urinary tract infection
She was admitted to the paediatric ward where she was rehydrated, transfused with packed cells, and treated with analgesia, a topical antifungal and empiric intravenous antibiotics. Within 5 days her diarrhoea resolved completely and she was discharged on oral amoxicillin clavulanic acid and an appointment for the ARV clinic.
Two weeks later she was stable, having gained 1 kg. The mother still complained of persisting lethargy in the child. Examination revealed a non-tender hepatosplenomegaly and right-sided suppurative otitis media. She was started on ARVs stavudine, lamivudine and nevirapine.
Currently, four weeks after starting ARVs she presented with a one-week history of a right axillary swelling which was found to be 2.5 cm in diameter, hard, tender and non-fluctuant. It enlarged rapidly and within six days had reached approximately 4×3 cm.
Differential Diagnosis
- Pyogenic abscess
- Lymphoma
- Kaposi’s sarcoma
- Immune Reconstitution Inflammatory Syndrome (IRIS)
- BCG adenitis
- Lymph node tuberculosis
Examination
Vitals
- Pulse-98
- Respiratory Rate-30
- Temperature-37.0
- Blood Pressure- not recorded
Head and Neck
- Oral thrush
- No cervical lymphadenopathy
Respiratory System
- Chest is symmetrical in appearance, no scars.
- Trachea in the midline
- Clear on auscultation bilaterally.
Cardiovascular System
- Capillary refill within 2 seconds.
- Normal S1 and S2, no gallop, no murmurs
Abdomen
- Mild hepatosplenomegaly.
- Bowel sounds present
Derm
- Round skin lesion on left arm
Investigations
| Values | |
|---|---|
| White Cell Count | 9.53x10 9 /l |
| Haemoglobin | 10.7 g/dl |
| Platelets | 397x109/l |
| CD4 | %=6.41, absolute count=77 cells/ul |
| Viral Load | |
| Blood culture and Blood TB culture | Negative |
| Chest Radiograph | Normal |
| Tuberculin Skin Test | Negative |
| Gastric washing | AFB negative x3 |
| Cerebrospinal fluid | Normal cells and chemistry, low adenosine deaminase |
| Bone marrow trephine and biopsy | Features in keeping with HIV |
| Ziehl-Nielsen Stain | Negative |
| TB culture | Negative |
Histophathology Results:
Fine needle aspiration of Right Axillary Mass:
- Numerous lymphocytes, mature and immature, were seen, with several groups of epitheliod hystiocytes and scattered Langerhans giant cells.
- A single Reed-Sternberg-like cell was noted.
- There were no features of lymphoma or any other malignant infiltrate.
- Scattered acid-fast bacilli were shown on Ziehl-Neelsen stain.
Histology of partial excision biopsy:
- Multiple lymph nodes with granulomatous non-caseating inflammation
- Langerhans type giant cells
- Epithelioid histiocytes
- No features of Hodgkin’s Lymphoma, Kaposis’s or any other malignant infiltrate
Discussion
Although the exact mechanism involved in the development of immune reconstitution inflammatory syndrome (IRIS) is not clearly understood, the explanation presented in this discussion is based on current theories.
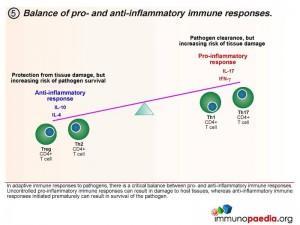
As a background to understanding inflammatory responses, it is first important to appreciate that there are four defined CD4+ T cell subsets that provide a homeostatic balance between pro and anti inflammatory responses that occur in a healthy functioning immune system. The four CD4+ T cell subsets we will discuss are the Th1, Th17, Treg and the Th2 subset.
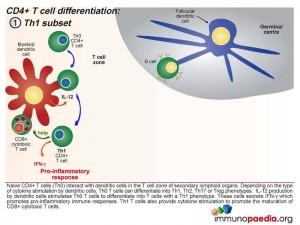
Initially, naive CD4+ T cells (termed Th0) encounter processed antigen by dendritic cells through the MHC class II and T cell receptor in the T cell zone of secondary lymphoid tissues, such as the lymph nodes. Depending on the type of cytokine released by the dendritic cell upon this interaction, the Th0 CD4 cells differentiate into either Th1, Th2, Th17 or Treg phenotypes.
Th1 Subset
Interleukin-12 released by the dendritic cells will cause the Th0 CD4 cells to differentiate into Th1 functional cells. These cells secrete IFN-γ which promotes pro-inflammatory responses and will mobilize the maturation of CD8+ cytotoxic T cells and stimulate NK cells.
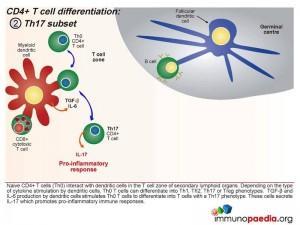
Th17 subset
When a combination of Transforming Growth Factor (TGF β) and IL-6 are released together by the dendritic cell, the Th0 CD4 cells differentiate into Th17 functional cells. These cells secrete IL-17, which also induces pro-inflammatory cytokines and tips the balance towards inflammatory immune responses.
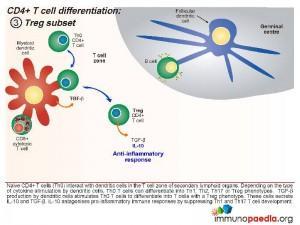
Treg Subset
When the dendritic cells only release TGF- β, the Th0 CD4 cells differentiate into T regulatory (Treg) cells. These cells secrete IL-10 and TGF- β. IL-10 antagonises pro-inflammatory immune responses by suppressing Th1 and Th17 T cell development.
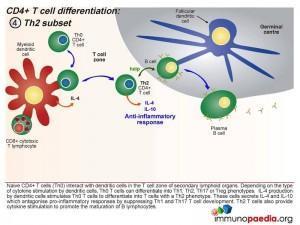
Th2 Subset
When the dendritic cells release IL-4 after Th0 binding, the cells differentiate into Th2 cells. These cells secrete IL-4 and IL-10 which antagonise pro-inflammatory responses by suppressing Th1 and Th17 T cell development. Th2 T cells also provide cytokine stimulation to promote the maturation of B cells to plasma cells and the production of antibodies.
Homeostatic balance of pro- and anti-inflammatory immune responses.
In adaptive immune responses to pathogens, there is a critical balance between pro- and anti-inflammatory immune responses. Uncontrolled pro-inflammatory immune responses can result in damage to host tissues, whereas anti-inflammatory immune responses initiated prematurely can result in survival of the pathogen, which is deleterious for the host. Th1 and Th17 CD4+ T cell subsets result in pro-inflammatory responses and Treg and Th2 CD4+ T cell subsets result in anti-inflammatory responses. It is thought that in a natural healthy immune response, there is a homeostatic balance between these subsets, resulting in the elimination of the pathogen, but also reducing the risk of tissue damage in the process. Inability to clear pathogen, or an overload of antigen, is either a result of this balance or a cause of an imbalance.
What happens when IRIS occurs?
Currently, it is thought that IRIS is a result of an imbalance towards pro-inflammatory immune responses during the immune reconstitution phase that occurs shortly after initiation of ARV therapy. There is a preferential differentiation of pro-inflammatory CD4+ T cell subsets.
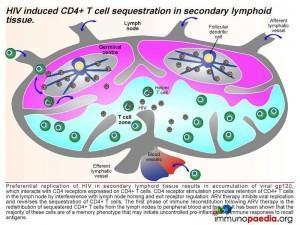
If we break down the sequence of events: when a person becomes infected with HIV, the virus replicates preferentially in secondary lymphoid tissue.
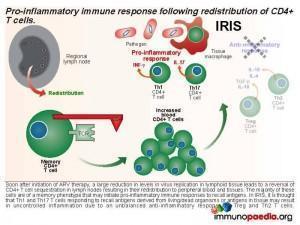 This results in an accumulation of viral gp120, which interacts with CD4 receptors expressed on CD4+ T cells and promotes retention of CD4+ T cells in the lymph node by interference with lymph node homing and exit receptor regulation. When ARV therapy is initiated, the drugs act by inhibiting viral replication and allow the release of sequestrated CD4+ T cells. Thus, the first phase of immune reconstitution following ARV therapy is the redistribution of sequestered CD4+ T cells from the lymph nodes to peripheral blood and tissues. It has been shown that the majority of these cells are Th1 and Th17 memory cells and the second phase is the resulting imbalance towards inflammatory responses due to these cells initiating uncontrolled pro-inflammation.
This results in an accumulation of viral gp120, which interacts with CD4 receptors expressed on CD4+ T cells and promotes retention of CD4+ T cells in the lymph node by interference with lymph node homing and exit receptor regulation. When ARV therapy is initiated, the drugs act by inhibiting viral replication and allow the release of sequestrated CD4+ T cells. Thus, the first phase of immune reconstitution following ARV therapy is the redistribution of sequestered CD4+ T cells from the lymph nodes to peripheral blood and tissues. It has been shown that the majority of these cells are Th1 and Th17 memory cells and the second phase is the resulting imbalance towards inflammatory responses due to these cells initiating uncontrolled pro-inflammation.
Download images for this case
Treatment
A course of amoxicillin clavulanic acid and metranidazole was prescribed for the differential of an abscess and clotrimazole for the skin lesion. Despite treatment, the right axillary mass continued to rapidly enlarge to a size of more than 10 cm.
Download images for this case
Final outcome
- Mycobacterium bovis was eventually cultured from the fine needle aspiration.
- Anti TB treatment was started.
- Nevirapine was switched to efavirenz to prevent drug interactions – specifically hepatotoxicity
Download images for this case
References
Sun HY et al. (2009). Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis. Aug;22(4):394-402. Review.
Elston JW et al. (2009) Immune reconstitution inflammatory syndrome. Int J STD AIDS. Apr;20(4):221-4. Review.
Mori S et al. (2009). A brief review of potential mechanisms of immune reconstitution inflammatory syndrome in HIV following antiretroviral therapy. Int J STD AIDS. Jul;20(7):447-52. Review.
Download images for this case
Evaluation – Questions & answers
What is the diagnosis in this case?
In this specific case presentation, there was an acute lymphadenopathy ipsilateral to the BCG inoculation site occurring 4 weeks after initiation of HAART, which is fairly typical of BCG IRIS. In children, IRIS resulting from BCG may occur months to years after vaccination. Most occur within the first 3 months of starting HAART and can be as early as 1 week after initiation of HAART. Less commonly, IRIS can manifest as an occurrence of non-infectious disease, such as Guillain-Barré syndrome.
What is immune reconstitution inflammatory syndrome (IRIS)?
What factors make a patient more likely to develop an IRIS event?
In what time frame does an IRIS event occur?
How does IRIS occur?
Who should get BCG vaccinations?
BCG is on the current South African EPI and is given at birth
Should antiretroviral therapy be stopped or continued if IRIS is suspected/confirmed?
Should the patient be treated for TB?
Should her ARV’s be stopped in the face of rapidly enlarging lymphadenopathy?
Who is at risk of an IRIS reaction?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case