- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigation
- Discussion
- Treatment
- Final Outcome
- Evaluation - Questions & Answers
- MCQ
Patient Presentation
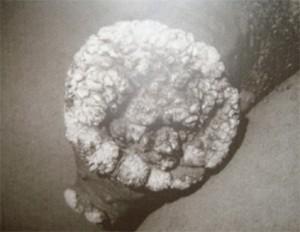
A 28 year old HIV-infected man presented to a Sexually Transmitted Infections (STIs) clinic with a large cauliflower like wart on the shaft of his penis. The wart had appeared four years previously and had been getting progressively larger. Although he had accessed healthcare on one occasion when the lesion initially appeared he had not followed up for any further care or treatment.
Acknowledgment: Federation of Infectious Diseases Societies of South Africa
History
Patient initially presented to his local clinic four years ago when he noted a small painless lesion on the shaft of his penis that had been present for about a month. At this visit he was counseled and tested for HIV.
At this visit he was found to be HIV positive. Additional tests were ordered for ELISA and CD4 count. He was asked to return in two weeks for his blood results.
ELISA confirmed he was HIV positive and CD4 was >350 cells/ul
However he never returned for his results and was lost to follow up until now.
Past Medical History
- Nil
Past Surgical History
- Nil
Family History
- Unknown
Allergies
- Unknown
Medication
- Not yet initiated on ARVs, patient not ready to start treatment
Travel History
- Nil
Social history
- Unemployed
- Smokes cigarettes, (unable to quantify) and social alcohol consumption.
- Unmarried, but is sexually active. States that he has used condoms with all of his sexual partners in the last four years.
Diagnosis
- Bowen disease
- Genital warts
- Giant Condyloma of Buschke and Lowenstein (GCBL)
- Seborrheic keratoses
- Squamous cell carcinoma
Examination
Appearance
- Ill looking male, average height and underweight
- Awake, alert, and co operative
- GCS 15/15
Vitals
- Temperature: 37.0°C
- Blood pressure: 135/78
- Heart rate: 95
- Respiratory rate: 16
General
- Mild pallor
- No jaundice
- Small shotty lymphadenopathy in left inguinal area
- No oedema
Head and Neck
- No abnormalities noted
Chest
- Trachea centrally located
- No wheeze
- Lung fields clear bilaterally
- No other added breath sounds
Cardiovascular
- No raised JVP
- Normally placed apex beat
- S1 and S2 heart sounds present, no gallop, no murmurs.
- No abnormalities detected
Abdomen
- Not distended
- Soft, no generalised tenderness
- Bowel sounds present
- No abnormalities detected
- No organomegally
Neurological
- Normal level of consciousness
- Gait normal
- Power 5/5 globally
- Tone normal globally
- Reflexes 2/4 for both upper and lower limbs
- No signs of peripheral neuropathy
Genitourinary
- Shotty regional lymphadenopathy in left inguinal area
- Uncircumcised phallus
- Raised, voluminous, exophytic, cauliflower like mass on shaft of penis, 9 x 6 cm in size.
- No urethral discharge
- No bleeding
- No fistula or abscess formation noted
- No perianal lesions
- Testicles normal
| Examination | Result |
|---|---|
| RPR for syphilis | negative |
| Urine PCR for Trichomonas vaginalis | positive |
| PCR for Chlamydia trachomatis | negative |
| PCR for Neisseria gonorrhoea | negative |
| HIV ELISA | positive |
| CD4 | 74 cells/ul |
| CD8 | 521 cells/ul |
| Total CD3 | 716 cells/ul |
| CD4: CD8 ratio | 0.14000000000000001 |
| Biopsy of wart | Buscke Lowenstein tumor. |
| PCR of biopsy, positive for: | HPV-6, 11, 55, 58, 59, 68, 73, 83 and cp 6108 |
Discussion
His case explores the clinical manifestation of Giant Condyloma of Buschke and Lowenstein (GCBL), first described by Buschke and Löwenstein in 1925.
GCBL is a slow-growing, locally destructive verrucous plaque that typically appears on the penis but may occur elsewhere in the anogenital region. It is a rare sexually transmitted disease, affecting 0.01% of the general population. The virus responsible for GCBL is human papillomavirus (HPV), usually type 6 or 11 and occasionally 16, 18 and 45. In our case study the patient was shown to be infected by multiple HPV strains; HPV- 6, 11, 55, 58, 59, 68, 73, 83 and cp 6108. GCBL may occur at any age after puberty and is characterized by invasive growth. There is a high rate of recurrence after treatment with 20-30% of cases undergoing malignant transformation. Clinically, most cases of GCBL appear on the glans penis but may occur on any ano-genital mucosal surface, including the vulva, vagina, cervix, anus, scrotum and bladder. If untreated, GCBL can cause destruction of tissues extending into the pelvic organs. In the case presented, GCBL started on the prepuce as a plaque and slowly expanded into a cauliflower-like mass, reaching 9cm in length. GCBL lesions have been shown to grow as large as 15cm. Although neither malignant transformation nor additional complications occurred in this case, it is important to note that these lesions can form extensive local destruction, with ulcerations, bleeding, fistulation and undergo malignant transformation.
Risk factors and predisposing factors which promote HPV infection resulting in GCBL include chronic phimosis and poor penile hygiene. This may account for the higher incidence in males who are uncircumcised. Immunosuppression secondary to HIV infection or immunosuppressants is also a major predisposing factor. Other risk factors include multiple sexual partners, chronic genital infections and pregnancy (associated with an impaired immune response)
To understand how HPV infects mucosal surfaces to form GCBL and occasionally resulting in malignant transformation, it is important to first understand the pathophysiology and immune evasion strategies of HPV. All mechanisms described are carefully illustrated in stepwise graphics to assist with understanding. Click on each graphic to see an enlarged version.
Pathophysiology of HPV
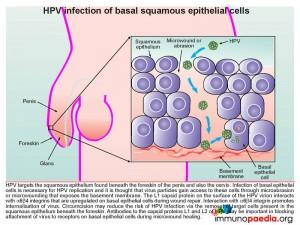
Human papillomavirus (HPV) targets the squamous epithelium found beneath the foreskin of the penis (illustrated), the scrotum, vulva, vagina, cervix, anus or bladder. Specifically the basal epithelial cell layer, the deepest epidermal layer, is necessary for HPV replication. The virus gains access to this depth of the squamous epithelium via micro-wounds and abrasions. During subsequent wound repair basal cells upregulate α6ß4 integrin which interacts with L1, a capsid protein, found on the surface of the HPV virion. This interaction promotes internalization of the virus. Evidence to support this process of HPV gaining cell entry has been shown by the use of antibodies targeting capsid proteins L1 and L2 that results in blocking attachment of virions to the basal epithelial cells during micro-wound healing.
Circumcision also reduces the risk of HPV infection, although the mechanism of protection is not clearly defined. The removal of the foreskin may decrease the occurrence of micro traumas to the squamous epithelial layer beneath the foreskin thereby decreasing the opportunity for HPV replication in this area. However infection can take place anywhere on the penis including the penile shaft, glans/corona and scrotum.
HPV replication in squamous epithelial cells
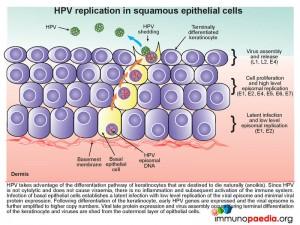
The epidermis, which is the outer layer of the skin, is made up of squamous epithelium. The epidermis is composed of 4-5 layers of cells; the cornified layer (stratum corneum), clear/translucent layer (stratum lucidum), granular layer (stratum granulosum), spinous layer (stratum spinosum), and the deepest layer the basal/germinal layer (stratum basale/germinativum). The predominant cell type is the keratinocyte, whose primary function is the formation of a barrier against environmental damage such as pathogens, heat, UV radiation and water loss. Keratinocytes differentiate as they move through the cell layers, starting as basal keratinocytes. Keratinization is part of the physical barrier formation, in which keratinocytes produce more and more keratin and eventually undergo natural cell death and detachment (anoikis). The fully cornified keratinocytes that form the outermost layer of the epidermis are constantly shed off and replaced by new cells. Squamous epithelium is avascular and nourished by diffusion from the underlying dermis.
HPV takes advantage of the differentiation pathway of keratinocytes and the fact that the cells die naturally with subsequent detachment, a process known as anoikis. Since HPV is not a cytolytic virus and does not cause viraemia, there is no inflammatory response initiated during HPV infection. Infection of the basal epithelial cells establishes a latent infection with low level replication of the viral episome and minimal viral protein expression (E1 and E2). As the keratinocyte differentiates, other early HPV genes are expressed (E1, E2, E4, E5, E6 and E7) and the viral episome is amplified with high level episomal replication. Finally during the terminal differentiation of the keratinocyte in the stratum corneum late viral protein expression (L1, L2) and virus assembly occurs. Thereafter, following the natural desquamation process where the outermost layer of epithelial cells are shed, virus is shed at the same time.
HPV immune evasion strategies
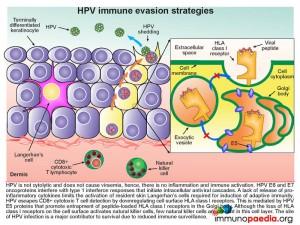
Although most people clear HPV and develop antibody responses, HPV can also employ several evasion strategies to avoid immune surveillance. These are listed below and illustrated in this graphic on the right.
- As this is not a cytolytic virus, there is no viraemia and no initiation of inflammation and subsequent activation of the immune system.
- HPV E6 and E7 oncoproteins interfere with type 1 interferon responses preventing the initiation of intracellular antiviral cascades.
- The lack of inflammatory cytokine release from infected keratinocytes limits the activation of resident skin Langerhan’s cells which are required for induction of adaptive immunity.
- The HPV E5 proteins promote entrapment of peptide-loaded HLA class I receptors in the Golgi body and there is thus a downregulation of HLA class l on the cell surface. This lack of HLA surface expression allows escape from virus-specific CD8+ cytotoxic T cell detection.
- Although the lack of HLA class I expression on the cell surface activates natural killer cells, few of these cells are resident or migrate to this cell layer.
- The anatomical location of HPV replication is an avascular area, which facilitates immune evasion due to inaccessibility of different cells of the immune system.
How is the Development of Carcinoma associated with HPV?
Of the 120 known types of HPV isolated from humans, 40 types are sexually transmitted and spread easily through the genital tract of men and women. Although in our case study, the wart was not malignant, a small group of HPV types are known as high-risk, oncogenic or carcinogenic and have been shown to cause penile, cervical, vulval and anal cancers. HPV types 16, 18, 31, 33, 35, 45, 52, and 58 are known to be amongst the high-risk oncogenic HPVs and are the most important types in cervical cancer. How does this occur? Persistent infections with these high-risk HPV types can cause cell abnormalities with the possibility of malignant transformation. Although penile cancer has a relatively rare occurrence, accounting for 0.2% of cancers, cervical cancer is one of the top five malignancies that kill women, and the leading cause of cancer-related deaths in South Africa. The patient in our case was identified to have several HPV types including the high-risk oncogenic HPV types- 6, 11 and 58, but was not shown on biopsy to have undergone malignant transformation.
The following series of graphics aims to explain the steps to development of squamous carcinoma, the commonest complication of infection with high-risk HPV types.
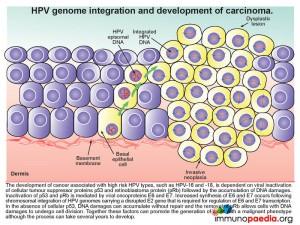
HPV genome integration and development of carcinoma
The development of cancer associated with high risk HPV types, such as HPV-16 and -18, is dependent on viral inactivation of cellular tumour suppressor proteins p53 and retinoblastoma protein (pRb) and the subsequent accumulation of DNA damages. Inactivation of p53 and pRb is mediated by viral oncoproteins E6 and E7. Increased synthesis of E6 and E7 occurs following chromosomal integration of HPV genomes carrying a disrupted E2 gene that is required for regulation of E6 and E7 transcription. In the absence of cellular p53, DNA damage can accumulate without repair and in the absence of pRB cells with DNA damage are able to undergo cell division. Together these factors can promote the creation of a cell with a malignant phenotype. The process is slow and can take several years to develop.
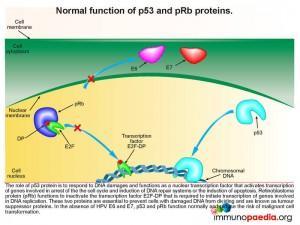 Lets look at this in closer detail. To better understand the link between HPV and malignancy, it is important to understand the events that are known to prevent malignant transformation. The normal role of p53 is to respond to damages occurring to DNA and to function as a nuclear transcription factor activating transcription of genes involved in arresting the cell cycle, induction of DNA repair systems or induction of apoptosis. Retinoblastoma protein (pRb) functions to inactivate the transcription factor E2F-DP required to initiate the transcription of genes involved in DNA replication. Together, p53 and pRb are essential in preventing cells with damaged DNA from dividing, thereby preventing malignant transformation, and are known as tumour suppressor proteins.
Lets look at this in closer detail. To better understand the link between HPV and malignancy, it is important to understand the events that are known to prevent malignant transformation. The normal role of p53 is to respond to damages occurring to DNA and to function as a nuclear transcription factor activating transcription of genes involved in arresting the cell cycle, induction of DNA repair systems or induction of apoptosis. Retinoblastoma protein (pRb) functions to inactivate the transcription factor E2F-DP required to initiate the transcription of genes involved in DNA replication. Together, p53 and pRb are essential in preventing cells with damaged DNA from dividing, thereby preventing malignant transformation, and are known as tumour suppressor proteins.
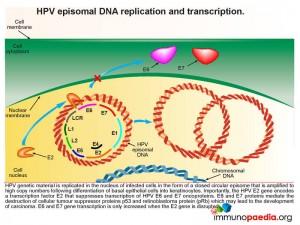
How does HPV disrupt the normal functions of p53 and pRb
When a squamous epithelial cell is infected with HPV, genetic material is replicated in the nucleus of infected cells in the form of a closed circular episome. This is amplified to high copy numbers during the differentiation process of basal epithelial cells into keratinocytes. Importantly, the HPV E2 gene encodes a transcription factor E2 that suppresses transcription of HPV E6 and E7 oncoproteins. E6 and E7 transcription can only be increased if the E2 gene is disrupted. When E6 and E7 transcription is increased they mediate the destruction of the cellular tumour suppressor proteins p53 and retinoblastoma protein (pRb) with a resulting possibility of development of carcinoma.
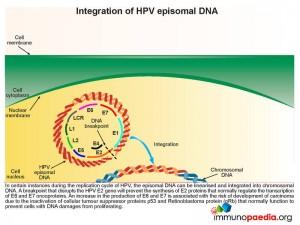
Integration of HPV episomal DNA
In certain instances during the replication cycle of HPV, the episomal DNA can be linearised and integrated into chromosomal DNA. A breakpoint that disrupts the HPV E2 gene will prevent the synthesis of E2 proteins that normally regulate the transcription of E6 and E7 oncoproteins. As has already been described this disruption of E2 results in an increased synthesis of E6 and E7 and the associated risk of carcinoma development.
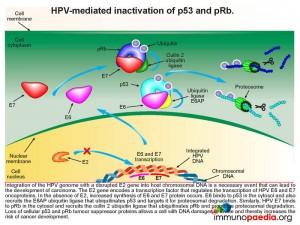
When there is an increased synthesis of E6 and E7, E6 binds to p53 in the cytosol and recruits the E6AP ubiquitin ligase that ubiquitinates p53 and targets it for proteosomal degradation. Similarly, HPV E7 binds to pRb in the cytosol and recruits the cullin 2 ubiquitin ligase that ubiquitinates pRb and promotes proteosomal degradation. Loss of cellular p53 and pRb tumour suppressor proteins allows a cell with DNA damages to divide thereby increasing the risk of cancer development.
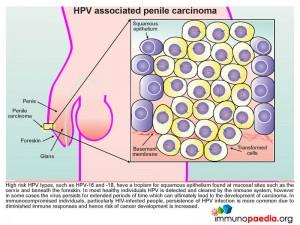
HPV associated penile carcinoma
High risk HPV types, such as HPV-16 and 18, have a tropism for squamous epithelium found at mucosal sites such as on the cervix and beneath the foreskin. In most healthy individuals HPV is detected and cleared by the immune system, however in some cases the virus persists for extended periods of time which can ultimately lead to the development of carcinoma. In immunocompromised individuals, particularly HIV-infected people, persistence of HPV infection is more common due to diminished immune responses and hence the risk of cancer development is increased.
Download images for this case
Treatment
Treatment and Prevention
Treatment
Giant Condyloma of Buschke and Lowenstein (GCBL) is typically a benign disease, but it carries a risk of malignant transformation. It generally develops slowly which results in delayed presentation, often patients will wait several years before seeking treatment, as demonstrated in our case study. It can also be locally destructive requiring medical and surgical interventions.
The recommended treatments for GCBL include:
- topical application such as podophyllin and imiquimod (aldara), radiation therapy, cryotherapy and electrotherapy
- systemic immunotherapy
- systemic chemotherapy to reduce tumor mass
- surgery
Adjunct therapies in more advanced or recurrent lesions include:
- radiotherapy
- photodynamic therapy
- imiquimod (aldara), an immune response modifier primarily acting on IFN-α, IL-6 and TNF-α
The most important consideration in developing a treatment strategy is to decide which option will achieve the best clinical result with the least morbidity to the patient.
Inadequately treated GCBL has a relentless progression. By definition, adequately treated GCBL has a low recurrence rate and therefore an excellent prognosis. However, studies have shown that adequately treated perianal/anogenital GCBL have a 68% recurrence rate with a 21% mortality rate.
Therefore follow up of patients is important.
Prevention
- Early circumcision has been found to be extremely effective in preventing penile carcinoma. This may be due to the reduction of HPV infection via the removal of the squamous epithelial cell layer beneath the foreskin.
- Due to the association with HPV, condom use is effective in decreasing the incidence of GCBL.
- The HPV vaccine (Gardasil) is indicated for the prevention of condyloma acuminata due to HPV types 6,11, 16 and 18 in boys, men, girls, and women aged 9-26 years. The vaccine is administered as 3 separate doses administered at 0, 2, and 6 months.
The patient in this study was initially treated topically with cryotherapy, followed by four applications of 20% Podophyllin. His response to both topical treatments was good.
He was then scheduled for chemo-radiotherapy for complete resolution and to start ARVs.
Download images for this case
Final Outcome
Before starting ARVs or chemo-radiotherapy the patient was lost to follow up. No further information has become available regarding the outcome of this patient.
Download images for this case
Evaluation – Questions & Answers
What is the diagnosis?
What are the most common presenting signs and symptoms of GCBL?
What are the risk factors and predisposing factors which promote HPV infection resulting in GCBL?
- Chronic phimosis and poor penile hygiene.
- Uncircumcised males.
- Immunosuppression secondary to HIV infection, immunosuppressant’s or pregnancy
- Multiple sexual partners and chronic genital infections
What is the pathophysiology of HPV?
Human papillomavirus (HPV) targets the squamous epithelium found beneath the foreskin of the penis, the scrotum, vulva, vagina, cervix, anus or bladder. Specifically the basal epithelial cell layer, the deepest epidermal layer, is necessary for HPV replication. The virus gains access to this depth of the squamous epithelium via microwounds and abrasions. During subsequent wound repair basal cells upregulate α6ß4 integrin which interacts with L1, a capsid protein, found on the surface of the HPV virion. This interaction promotes internalisation of the virus.
HPV then takes advantage of the differentiation pathway of keratinocytes from basal keratinocytes to terminal differentiation in the stratum corneum, the most superficial layer of the squamous epithelium. During this differentiation process there is replication of the viral episome, which is amplified to large numbers of copies. Eventually when the cells reach the stratum corneum where the keratinocytes undergo natural cell death and are shed off, the virus is shed into the environment at the same time.
What is the pathophysiology of HPV?
Human papillomavirus (HPV) targets the squamous epithelium found beneath the foreskin of the penis, the scrotum, vulva, vagina, cervix, anus or bladder. Specifically the basal epithelial cell layer, the deepest epidermal layer, is necessary for HPV replication. The virus gains access to this depth of the squamous epithelium via microwounds and abrasions. During subsequent wound repair basal cells upregulate α6ß4 integrin which interacts with L1, a capsid protein, found on the surface of the HPV virion. This interaction promotes internalisation of the virus.
HPV then takes advantage of the differentiation pathway of keratinocytes from basal keratinocytes to terminal differentiation in the stratum corneum, the most superficial layer of the squamous epithelium. During this differentiation process there is replication of the viral episome, which is amplified to large numbers of copies. Eventually when the cells reach the stratum corneum where the keratinocytes undergo natural cell death and are shed off, the virus is shed into the environment at the same time.
How does HPV promote the development of carcinoma?
What are the immune evasion strategies used by HPV?
- As this is not a cytolytic virus, it does not cause viraemia with inflammation and subsequent immune activation.
- HPV E6 and E7 oncoproteins interfere with type 1 interferon responses preventing the initiation of intracellular antiviral cascades.
- The lack of release of pro-inflammatory cytokines limits the activation of resident skin Langerhan’s cells, which are required for induction of adaptive immunity.
- The HPV E5 proteins promote entrapment of peptide-loaded HLA class I receptors in the Golgi body. By downregulation of these cell surface HLA class l receptors, they escape virus-specific CD8+ cytotoxic T cell detection
- Although the loss of HLA class I receptors on the cell surface activates natural killer cells, few natural killer cells are present in this cell layer.
- The site of HPV replication is a major contributor to immune evasion because this is an avascular area with reduced immune surveillance.
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case