- Patient presentation
- History
- Differential diagnosis
- Examination
- Investigations
- Case Discussion
- Treatment
- Final Outcome
- Evaluation - Questions & answers
- MCQ
Patient presentation
A previously healthy 6–year-old boy is brought to the emergency room with a 3-day history of fever, neck stiffness and a one day history of a generalised maculopapular rash.
Acknowledgement
This case study was kindly provided by Dr Monika Esser MMed Paed, Head of Division of Immunology, N.H.L.S Coastal Branch, Tygerberg Hospital.
History
A 6-year-old boy, previously healthy, presents with a 3-day history of progressively worsening fever, headache, neck stiffness and general malaise. In the last day he has developed a maculopapular rash which started on the extremities and is now also on the trunk. He has been complaining of nausea but no vomiting and his appetite has decreased.
Past medical and surgical history
No significant medical or surgical history. Road to health card shows all growth parameters to be within normal limits with all vaccinations up to date.
Family and social history
He lives with his mother, father, and one younger sibling who are all healthy. His mother was recently tested and is HIV negative. Father’s status is unknown. They live on a farm in the Western Cape with a small number of livestock that includes cattle, sheep and goats.
Travel history
No travel outside of Western Cape in the last 12 months.
Differential diagnosis
- Meningitis
- Chicken Pox
- Tick Bite fever
- Measles
- Enteroviral infection
Examination
Vitals
- Pulse – 130
- Respiratory Rate – 34
- Temperature – 39.8
General
Inguinal lymph node enlargement
Chest
- Chest shape normal in appearance, tachypnoea present
- Midline trachea
- Clear on auscultation bilaterally
Cardiovascular
- Tachycardia with a regular rhythm
- Normal S1 and S2, no murmurs
- Bounding pulses felt radially and femorally
- Capillary refill within 2 seconds
Abdomen
- Normal on inspection.
- No rigidity or guarding.
- No organomegally
- Bowel sounds present
Neurological
Fully awake, alert and co operative
Dermatological
- Maculopapular rash with non-blanching petechial centers found in the lesions. Distribution is on arms, legs and trunk
- No bite marks or eschars seen
Investigations
| Examination | Value | Normal Limits |
|---|---|---|
| WBC | 8 | (4-12 x 109/l) |
| HB | 12 | (12.1-15.2 g/l) |
| Platelets | 222 | (140-450 x 109/l) |
| C-reactive protein | 50 | (0-8 mg/l) |
| PCR – R.conorii | Negative | |
| Indirect immunofluorescence – R.conorii | Netavie |
Discussion
 Rickettsial infection
Rickettsial infection
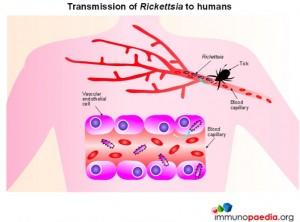
Members of the Rickettsia genus are Gram negative, obligate, intracellular bacteria with a life cycle that involves both an arthropod vector and a vertebrate host. Mammals constitute the principle reservoir with humans considered incidental hosts. Humans become infected by Rickettsial bacteria by direct inoculation from infected ticks.
There are over 30 different Rickettsial species that are generally classified into 4 groups based on their biological, genetic and antigenic characteristics. The groups include the spotted fever group (SFG), typhus group, transitional group and ancestral group. The spotted fever group is of major importance and includes the highly pathogenic organisms R.ricketsii, which causes Rocky Mountain Spotted Fever, R. conorii, which causes Mediterranean spotted fever and R.africae, the causative organism of African tick-bite fever.
Previously R.conorii was thought to be the only cause of South African tick-bite fever however in 1992 R.africae was identified as an alternative cause.
 Rickettsial Host Interactions
Rickettsial Host Interactions
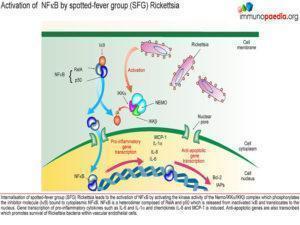
Following a tick bite which causes direct inoculation into the blood vessels, Rickettsial bacteria penetrate the vascular endothelial cells by a receptor-mediated endocytic pathway. Rickettsial bacteria express Outer membrane protein B (rOmpB) which binds to host cell receptor Ku70 expressed on the surface of vascular endothelial cells. Via an enzymatic cascade, actin is polymerized which is necessary for the internalisation of the bacteria.
The cell membrane then invaginates and phagocytosis of the bacterium occurs resulting in a phagosome.
The cell membrane of the phagosome is lysed by the secretion of bacterial membranolytic proteins: phospholipase D and haemolysin C. This occurs before the phagosome fuses with a lysosome for degradation thereby permitting the bacteria to escape into the cytosol.
Movement within the cytosol is achieved by actin polymerisation at one of the poles of the bacterium due to the bacterial protein RickA. The actin filaments produced are long unbranched actin chains which grow and allow the bacteria to move through the cell . The bacteria are then also able to penetrate adjacent cells through plasma membranes.
Pathogenesis
The basic pathology of Rickettsial disease is that of a multi-organ vasculitic process that causes an increase in microvascular permeability. However, whole organ failure is uncommon because the vasculitis occurs focally.
The bacteria also stimulate the major transcription factor NFκB in endothelial cells which induces pro-inflammatory cytokine production, including IFN-γ, TNF-α, IL-1β, IL-6 and IL-8. There is also inhibition of endothelial cell apoptosis leading to prolonging bacterial survival.
Haemostasis can also occur, which results in thrombocytopaenia. Endothelial injury can activate the coagulation cascade which will put the patient into a procoagulant state rarely resulting in DIC or haemorrhage.
 Host defense and immunity
Host defense and immunity
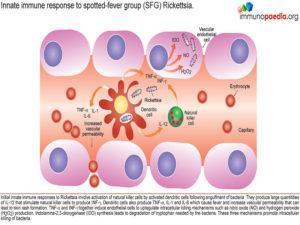
Initial innate immune responses to Rickettsia involve activation of natural killer cells by activated dendritic cells following engulfment of bacteria. They produce large quantities of IL-12 that stimulate natural killer cells to produce INF-γ. Therefore, during the early stages of infection, there are increased numbers of NK cells. The pro-inflammatory cytokines IFN-γ, TNF-α, IL-1β and IL-6 cause fever and increased vascular permeability resulting in a maculopapular rash with non-blanching petechiae. The cytokines also activate the infected endothelial cells to kill Rickettsiae via two autophagic mechanisms – nitric oxide and hydrogen peroxide. A third mechanism is Indolamine-2,3-dioxygenase (IDO) synthesis which leads to degradation of tryptophan needed by the bacteria to survive. The three mechanisms promote intracellular killing of bacteria.
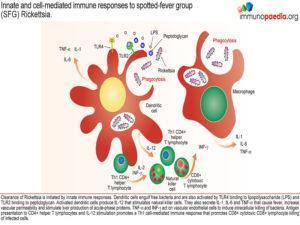 The clearance of Rickettsia is initially mediated by innate immune responses, where dendritic cells engulf free bacteria and also activate Toll Like Receptor (TLR) 4 with lipopolysaccharide (LPS). Activated dendritic cells then produce IL-12 that stimulates natural killer cells. They also produce the above mentioned cytokines that cause fever, increase vascular permeability and stimulate liver production of acute-phase proteins especially by IL-6.
The clearance of Rickettsia is initially mediated by innate immune responses, where dendritic cells engulf free bacteria and also activate Toll Like Receptor (TLR) 4 with lipopolysaccharide (LPS). Activated dendritic cells then produce IL-12 that stimulates natural killer cells. They also produce the above mentioned cytokines that cause fever, increase vascular permeability and stimulate liver production of acute-phase proteins especially by IL-6.
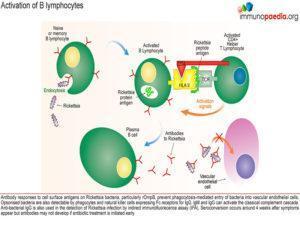
Humoral immunity forms late in the course of primary infection but confers immunity against re-infection.
 Clinical manifestations of South African Tick bite fever
Clinical manifestations of South African Tick bite fever
- Incubation period 5 – 7 days
- Fever, Malaise, Headache, Myalgia
- Eschar, an ulcerated lesion with central necrosis and surrounding inflammation
- Generalised maculopapular rash
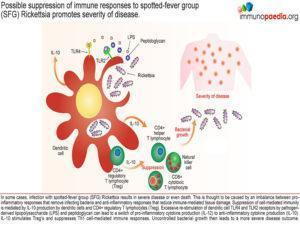
Occasionally infection with a SFG Rickettsia strain can lead to severe disease or even death. This is thought to be a result of an imbalance between the pro-inflammatory responses that remove infecting bacteria and suppression of the immune response to avoid immune-mediated tissue damage. Suppression of cell-mediated immunity often occurs following excessive stimulation of TLR4 and TLR2 on dendritic cells which is thought to cause a switch from IL-12 to IL-10 production and ensuing immune suppression. Should this suppression occur before adequate clearance of bacteria is achieved, uncontrolled levels of bacterial growth may then lead to severe disease or death.
- Clinical triad of fever, rash and an eschar in 50 – 75% of patients
- Confirmation of clinical diagnosis is usually delayed, therefore infections are typically treated empirically
- 3 Diagnostic tests are available- Serology, PCR techniques and Culture
Serology
- Most commonly used diagnostic tool.
- Immunofluorescence technique (IFA).
- Weil-Felix agglutination obsolete due to decreased sensitivity and specificity.
- Antigen derived from R. rickettsii
- Serology Pitfalls
- Cross reactivity within spotted fever group precludes speciation.
- Intra-group cross reactivity occurs at lower titers than group specific titers.
- Seroconversion occurs around 4 weeks after symptoms appear
- but antibodies may not develop if antibiotic treatment is initiated early. In this case the likely cause for the negative test results was that the patient was successfully treated with Doxycline i.e. there was no antibody response due to early treatment administration.
- Some patients remain IgM positive for prolonged periods.
- Caution in interpreting positive IgM in the absence of positive IgG.
- Tests are not available for detection of R. africae, beware of false negatives
PCR
- Blood or skin biopsy (eschar).
- Rapid, sensitive and specific.
- Not freely available.
Culture
- Intracellular bacteria.
- Isolate on cell culture.
- Seldom used: Difficult & Hazardous.
Download images for this case
Treatment
- No large trials have been conducted to examine optimal treatment strategies
- Treatment of choice: Doxycycline 100mg 12hrly for 5 – 7 days (3 – 14 days)
- Patient should respond within 48 hours, otherwise reconsider the diagnosis
- Children/Pregnancy: Doxycycline for 48 hrs followed by macrolide.
Download images for this case
Final Outcome
The patient was diagnosed clinically with tick-bite fever (TBF) and was initially managed with Clarithromycin. This was later changed to Doxycycline with excellent response and the patient made a full recovery.
Download images for this case
Evaluation – Questions & answers
What is the diagnosis?
What receptors allow Rickettsial bacteria to attach to endothelial cells?
What process is needed for the bacteria to become internalised?
Once a phagosome enters the cell, how does it prevent degradation from fusing with a lysosome?
How do bacteria move from cell to cell?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case