- Patient Presentation
- History
- Differential diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & answers
- MCQs
Patient presentation
Peter, a 60 year-old Caucasian policeman, complains of a painful burning sensation in his lower extremities lasting for several months. Lower limbs petechiae (small purple/red hemorrhagic spots) appeared one week ago.
Acknowledgement
This case study was provided by Prof. Olivier Boyer (M.D., Ph.D., Head of the Department of Immunology and Biotherapy, Rouen University Hospital, France) and Dr. Maëlle Le Besnerais (M.D., Assistant Professor of Internal Medicine, Rouen University Hospital, France) of the Faculty of Medicine of Rouen, Normandy University, France. The authors would like to thank David Saadoun, Odile Goria, Lucie Guyant-Maréchal and Fabienne Jouen for their critical reading of this case study, Isabelle Duval for the development of pictures and Laetitia Demoulins for technical assistance. We are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
History
Peter complains of chronic fatigue and aching joints which started several months ago. He denies significant alcohol consumption and intravenous drug abuse. He received a blood transfusion after a gunshot injury to his arm 35 years ago. He reports distal paraesthesia (tingling or numbness) of both legs and painful burning in both feet which has progressed to the lower and upper limbs. Knee pain wakes him up at night.
Past medical history
- None
- No allergies
Surgical history
- Appendix removed at 10 years old
- Arm gunshot injury 35 years ago
Family history
- His father has hypertension and type 2 diabetes
Travel history
- He traveled to Thailand 25 years ago
Social history
- Policeman, married, two children
Medication
- None
Differential diagnosis
- IgA vasculitis
- Polyarteritis nodosa
- ANCA-associated vasculitis (eg. granulomatosis with polyangiitis [formerly Wegener’s], microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis [formerly Churg and Strauss syndrome])
- Vasculitis associated with an autoimmune disease (eg. systemic lupus erythematosus, rheumatoid arthritis, Sjögren’s syndrome)
- Infection (eg. rickettsial infections, malaria, babesiosis)
- Infection related vasculitis (eg. bacterial endocarditis, poststreptococcal vasculitis and glomerulonephritis, Hepatitis C virus (HCV) associated mixed cryoglobulinemia vasculitis)
- Serum sickness
- Drug-induced small vessel vasculitis (hypersensitivity vasculitis)
Examination
Vitals
- Heart rate: 83/min
- Blood pressure: 114/72 mmHg
- Temperature: 37.8°C
- Oxygen saturation: 97%
General
- He looks tired
- No pallor
- Livedo reticularis (mottled reticulated vascular pattern that appears as a lace-like purplish discoloration of the skin)
- Symmetrically distributed palpable purpura (red/purple discolored spots on the skin that do not blanch on applying pressure) in the lower extremities
- Bilateral supramalleolar (above the ankle joint) skin ulcers
Cardiovascular
- Normal heart sounds
- All pulses present
Respiratory
- No dyspnea (shortness of breath), no cough
- Respiratory rate 17/min
- No chest deformity
- Good bilateral air entry
- No crackling sound on the lungs or wheezing
Abdomen
- Mild hepatomegaly (enlargement of the liver)
Neurological
- Normal cognition
- No meningism
- No paralysis of cranial nerves
- Normal motor strength
- Superficial hypoesthesia (decreased sensitivity to stimuli) of lower limb under knees associated with dysesthesia (unpleasant, abnormal sense of touch)
- Loss of joint position and vibration sense and sensory ataxia (failure of muscle coordination)
- Tendon reflexes were absent at the ankles bilaterally and reduced at the knee
- No sphincter disorder
Investigations
| Examination | Value | Normal limits |
|---|---|---|
| White blood cell | 7.41 | (4-12 x109/L) |
| Hemoglobin | 10.9 | (12.1-15.2 g/L) |
| Platelets | 175 | (140-450 x109/L) |
| C reactive protein (CRP) | 5 | (0-8 mg/l) |
| Sodium | 136 | (135-147 mmol/L) |
| Potassium | 4.2 | (3.3-5.0 mmol/L) |
| Urea | 6.2 | (2.5-6.4 mmol/L) |
| Creatinine | 96 | (62-115 mmol/L) |
| Creatinine kinase | 183 | (25-195 IU/L) |
| Total protein | 62 | (60-80 g/L) |
| Albumin | 37 | (35-50 g/L) |
| Corrected calcium | 2.35 | (2.1-2.6 mmol/L) |
| Phosphate | 1.3 | (1.0-1.5 mmol/L) |
| Magnesium | 1.1 | (0.8-1.3 mmol/L) |
| Aspartate aminotransferase | 56 | (10-35 IU/l) |
| Alanine aminotransferase | 70 | (10-35 IU/l) |
| γ-glutamyl transferase | 86 | (10-38 IU/l) |
| Alkaline phosphatase | 127 | (35-105 IU/l) |
| Bilirubin | 18 | (2-18 µmol/l) |
| Prothrombin time | 85 | (75-100%) |
| Thyroid stimulating hormone | 11.3 | (9-30 mIU/L) |
| Antinuclear antibodies | Negative | |
| Autoimmune hepatitis-specific antibodies (anti mitochondrial, anti smooth muscle, anti-LKM, anti-LCI) |
Negative | |
| Anti neutrophil cytoplasmic antibodies | Negative | |
| (anti-Myeloperoxydase (MPO) and anti Proteinase 3 (PR3)) |
||
| Anti-citrulline antibodies | Negative | |
| CH50 | 50 | (85-140 IU/ml) |
| C3 | 0.46 | (0.5-1.53 g/L) |
| C4 | 0.02 | (0.2-1 g/L) |
| Rheumatoid factor | 459 | <20 IU/ml |
| Serum protein electrophoresis | Hypogammaglobulinemia | |
| Cryoglobulin | See below | |
| Proteinuria | 0.09 | <0.15 g/24h |
| HIV | Negative | |
| HBs antigen | Negative | |
| Anti-HBs antibodies | Negative | |
| Anti-HBc antibodies | Negative | |
| Anti-HCV antibodies | Positive | |
| HCV polymerase chain reaction | 1.12x107 | (<15 IU/ml) |
| HCV genotyping | 1b |
| Cryoglobulin Examination | ||
|---|---|---|
| type III (polyclonal IgG, IgM κ/λ) | 2670 | (<50 mg/l) |
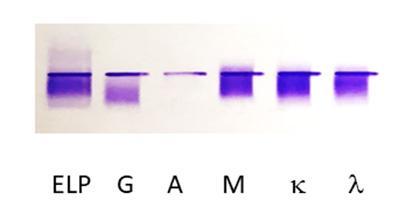
Figure 1: Immunofixation electrophoresis of Peter’s cryoglobulinemia. The first lane is protein electrophoresis (ELP). The next lanes are immunofixation performed with anti-heavy chain antibodies for IgG (G), IgA (A), IgM (M), and anti-light chain antibodies for kappa (κ) and lambda (λ). A type III polyclonal IgG, IgM κ/λ cryoglobulin is observed.
EKG: normal
Chest X-ray: normal
Computed tomography: mild hepatomegaly
Evaluation of liver fibrosis by Fibroscan: 8 kPa (moderate fibrosis without cirrhosis)
EMG: electromyography shows a severe length-dependent axonal sensitive polyneuropathy of the lower limbs
Skin biopsy: leukoclastic vasculitis with fibrinoid necrosis and thickening of the vessel wall associated with inflammatory infiltrate consisting of neutrophils as well as destruction of neutrophils, nuclear dust, and neutrophilic debris
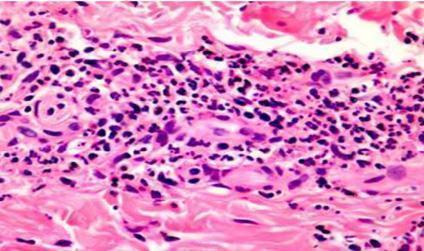
Figure 2: Histological analysis of a skin biopsy (H&E staining) showing leukoclastic vasculitis with fibrinoid necrosis associated with inflammatory infiltrate. [Jordan A. et al (2011). Pathology of the Cutaneous Vasculitides: A Comprehensive Review, Advances in the Etiology, Pathogenesis and Pathology of Vasculitis]
Discussion
The cause of the presenting condition is Hepatitis C Virus (HCV) associated mixed cryoglobulinemia vasculitis.
Cryoglobulins are protein in nature and can be defined by the presence of circulating immune complexes that precipitate as serum is cooled below core body temperature, and that resolubilize when serum is rewarmed. Cryoglobulins can comprise of a mixture of immunoglobulin (Ig) and complement components or just immunoglobulins only. (Bhandari, Awais and Aeddula, 2022)
Cryoglobulinemia refers to a condition with circulating cryoglobulins in the serum. Cryoglobulinemia vasculitis refers to small to medium size vessel vasculitis resulting from the pathogenicity of cryoglobulin-containing immune complexes.
The prevalence of clinically significant cryoglobulinemia is estimated at around 10 per million. Mixed cryoglobulinemia vasculitis more frequently occurs in patients aged 45–65 years, mainly in women (sex ratio women:men is 2–3:1) with no predominant ethnicity.
Significant proportions of patients with chronic infection or autoimmune diseases have detectable levels of cryoglobulins in their serum (about 40-65% of HCV infection, 15-20% of HIV infection, 15-25% of autoimmune diseases).
The different types of cryoglobulins
Classification of cryoglobulinemia is based on the clonality and type of immunoglobulins.
Classification
- Type I (monoclonal): isolated monoclonal lg (typically IgG or IgM, and less commonly lgA or free light chains), and found in conditions like monoclonal gammapathy of unknown signification, multiple myeloma and Waldenstrom’s macroglobulinemia. It accounts for 10-15% of all cryoglobulinemias.
- Type II (mixed): mixture of monoclonal IgM and polyclonal IgG, with the IgM component having positive rheumatoid factor activity. Also called essential mixed cryoglobulinemia and the term ‘essential’ arises from the fact that up to 10% of cases have no identifiable diseases associated to it. It has, however mostly been found in patients with chronic hepatitis C, autoimmune diseases (Sjögren’s syndrome, Lupus etc.), HIV, Hepatitis B virus (HBV)and B-cell lymphoma. This is the most common type and accounts for 40-60% of all cryoglobulinemias.
- Type III (mixed, polyclonal): mixed cryoglobulinemia consisting of polyclonal IgG and polyclonal IgM. Also a common type, mostly in association with autoimmune diseases and sometimes associated with infections (most common being HCV), accounting for 25-30% of all cryoglobulinemias.
Both type II and type III are referred to as mixed cryoglobulinemia since they both consist in a mixture of IgM and IgG. Typically, type III precedes type II during disease evolution.
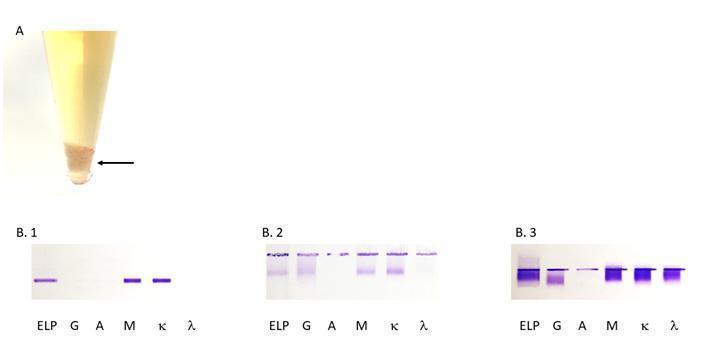
Figure 3: (A) Aspect of a cryoprecipitate at +4°C (arrowed), (B) the different types of cryoglobulins as determined by immunofixation. The first lane is protein electrophoresis (ELP). The next lanes are immunofixation performed with anti-heavy chain antibodies for IgG (G), IgA (A), IgM (M), and anti-light chain antibodies for kappa (κ) and lambda (λ). (B.1) type 1 IgM κ, (B.2) type 2 polyclonal IgG and IgM and monoclonal IgM κ, (B.3) type 3 polyclonal IgG and IgM (κ/λ).
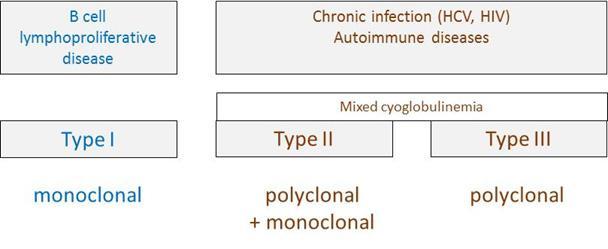
Figure 4: Types of cryoglobulins and their main causes. Type I and type II/III cryoglobulins result from different clinical contexts, i.e. B cell lymphoproliferation versus infection/autoimmunity, respectively.
Cryoglobulins: clinical spectrum
Type II and type III cryoglobulinemias are usually associated with constitutional and non-specific symptoms, such as arthralgias, fatigue and myalgias, as well as palpable purpura, cutaneous vasculitis (referred to as mixed cryoglobulinemia vasculitis) and peripheral neuropathy. Here, Peter displays the classical “Meltzer’s triad”, i.e. purpura, arthralgias and weakness, observed only in 25-30% of patients.
Dermatological involvement is observed in 90% of patients, consisting mainly in purpura but skin ulcers may be observed in 15 to 30% of patients. Other findings include Livedo reticularis, Raynaud’s phenomenon (digital ischemia) and necrosis. Symmetrical inflammatory arthralgias (usually involving hand joints, wrists and knees) without evidence of bone erosion affect 80% of patients. Peter developed Livedo reticularis and symmetrically distributed palpable purpura were observed in the lower extremities associated with two skin ulcers over the supramalleolar areas.
Patients with cryoglobulinemia commonly suffer from peripheral neuropathy. This complication frequently manifests as a distal, predominantly sensory polyneuropathy, with neuropathic pain being a frequently reported symptom. Here, Peter’s EMG evidenced polyneuropathy. Axonal sensitive polyneuropathy is the most common feature but multiplex mononevritis is also described.
As in other immune complex-associated diseases, renal involvement may occur. Most renal biopsies show membranoproliferative glomerulonephritis with immune cell infiltration, glomerular thrombi, and microtubular deposits composed of the cryoglobulin aggregates. Involvement of organs other than the kidneys is rare.
Cryoglobulins: diagnosis
The biological diagnosis of cryoglobulinemia is based on the demonstration that cryoglobulins are present in the serum.
Appropriate sample collection and handling is crucial to avoid a false negative. Samples should be transferred and centrifuged at 37°C to avoid precipitation before serum extraction. The serum is then refrigerated (4ºC) to allow the precipitation of cryoglobulins. Samples should be examined for 7 days because precipitation can be delayed. After cryoprecipitation, the precipitate is washed in a cold buffer and dissolved in a warm solution. Electrophoresis and immunofixation are then performed to determine the type of cryoglobulin. When cryoglobulins are detected, measurement of the cryocrit (i.e., the relative volume of the precipitate as a percentage of the total serum volume) should be reported if possible. Alternatively, the abundance of cryoprecipitate can be evaluated by measuring its protein concentration (milligram per liter).
Cryoglobulinemia is associated with the activation of rheumatoid factor-expressing B cells. As immune complexes, cryoglobulins contain IgM with rheumatoid factor activity, polyclonal anti-HCV IgG and antigen (virus) that precipitate and deposit on vascular endothelium. Assays of rheumatoid factor and complement (C3, C4, CH50) are part of the biological investigations of cryoglobulinemia. Complement, mainly C4 is decreased by consumption after activation of the classical pathway of complement.
It should be mentioned that when cryoglobulinemia is strongly suspected clinically, the negative result of a single routine laboratory test does not eliminate the possibility of mixed cryoglobulinemia vasculitis. The search for cryoglobulin can be repeated if the clinical suspicion is very strong. Additionally, many infections may be transiently accompanied by low levels of mixed polyclonal cryoglobulinemia.
The diagnosis of mixed cryoglobulinemia vasculitis typically requires:
- (1) the positivity of serum cryoglobulins in at least two determinations at 12 weeks interval or less
- (2) the positivity of some of the items below (questionnaire, clinical, laboratory)
- Questionnaire items (at least two out of the following three):
- Do you remember one or more episodes of small red spots on your skin, particularly involving the lower limbs?
- Have you ever had red spots on your lower extremities, which leave a brownish color after their disappearance?
- Has a doctor ever told you that you have viral hepatitis?
- Clinical items (at least three out of the following four, present or past):
- Constitutional symptoms: fatigue, low grade fever, fever (>38°C, no cause), fibromyalgia
- Articular involvement: arthralgias, arthritis
- Vascular involvement: purpura, skin ulcers, necrotizing vasculitis, hyperviscosity syndrome, Raynaud’s phenomenon
- Neurologic involvement: peripheral neuropathy, cranial nerve involvement, vasculitic central nervous system involvement
- Laboratory items (at least two out of the following three, present):
- Reduced serum C4
- Positive serum rheumatoid factor
- Positive serum monoclonal component (mainly IgM k)
Cryoglobulins: physiopathology
Peter suffers from chronic Hepatitis C. He was probably infected after the blood transfusion for his arm gunshot injury.
The mechanism of cryoglobulin pathogenicity is well described for HCV-associated cryoglobulinemia. HCV drives polyclonal B cell expansion, which induces a polyclonal anti-HCV antibody response. Some proliferating B cells also produce IgM with rheumatoid activity toward IgG. The rheumatoid factor (anti-IgG IgM) interacts with anti-HCV IgG to form immune complexes. Complement protein, C1q, binds these immune complexes that, in turn, bind to C1q receptors on endothelial cells. The deposits of immune complexes in the kidneys, small blood vessels and other organs promotes inflammatory cell recruitment and causes vasculitis. Inflammation causes the small blood vessels to burst and extravasate red blood cells into the skin, causing purpura. Arthritis may arise from deposition of immune complexes in the synovial joints.
Other diseases in which transient or permanent rheumatoid factor production may be observed include: rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, dermatomyositis, Sjögren’s syndrome, infectious mononucleosis, endocarditis, tuberculosis, syphilis, multiple myeloma, and Waldenstrom’s disease.
The pathophysiology of some autoimmune diseases may involve pathogenicity of immune complexes other than cryoglobulins. For instance: anti-DNA autoantibodies form immune complexes that deposit and activate complement in kidneys during systemic lupus erythematosus.
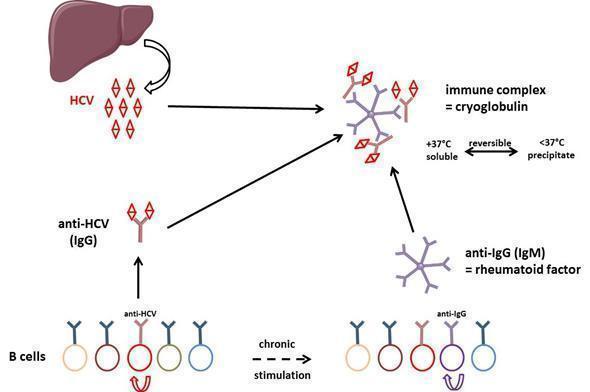
Figure 5: General mechanism of cryoglobulin production in HCV-infected individuals. Polyclonal anti-HCV antibodies bind to rheumatoid factor resulting in type III cryoglobulin. After some time of evolution, some B cell clones are overstimulated and type III cryoglobulin may evolve to type II cryoglobulinemia, i.e. production of a monoclonal component (not depicted in the figure) in addition to the polyclonal response.
Type III cryoglobulinemia results from chronic stimulation of B cell adaptive immunity: immune complexes are formed by HCV, anti-HCV polyclonal antibodies and rheumatoid factor. After electrophoresis and immunofixation of the cryoprecipitate, this results in a smear when revealed by anti-IgG and anti-IgM heavy and light chain specific antibodies. HCV is a hepatotropic but also lymphotropic virus. In the course of disease evolution, some B cells may become resistant to apoptosis because of expression of some viral genes, which results in clonal lymphoproliferation causing type II cryoglobulinemia. In this case, monoclonal components associate with the polyclonal Ig response, resulting in discrete bands after immunofixation with IgM and κ light chain antibody in most cases. A subset of patients may develop a low-grade lymphoma due to the transformation of the expanded B cells. HCV-related B cell lymphoproliferative disorders likely comprise a spectrum of diseases, ranging from asymptomatic clonal B cell expansions to pathogenic cryoglobulinemia and lymphoma.
Mixed (type II and III) cryoglobulins are different from type I cryoglobulins that develop in the setting of monoclonal gammopathies. The monoclonal component undergoes crystallization and aggregation, which is dependent on temperature and concentration.
Download images for this case
Treatment
Patient’s treatment plan
Peter’s proposed treatment was with direct antiviral agents but unfortunately he was lost to follow-up.
Recommended treatments
The therapeutic management of mixed cryoglobulinemia vasculitis must be individualized according to the underlying disorder and the severity of disease.
The majority of patients with mild and slow progressive disease will usually do well and no specific therapy is recommended in addition to that of the underlying disease.
In patients requiring therapy for cryoglobulinemia, the objective is to obtain minimal symptoms without side effects. There is a poor correlation between the abundance of the cryoglobulin (determined as cryocrit or protein concentration) and the response to treatment, whereas rheumatoid factor and complement studies (C3, C4, CH50) can be used to monitor the response to therapy.
Antiviral therapy
Antiviral therapy against HCV is the mainstay of treatment for cryoglobulinemic disorders associated with HCV infection. A combination of pegylated interferon-α plus ribavirin was recommended for many years. Direct-acting antiviral agents are more potent than the pegylated interferon-ribavirin combination, and are given orally, for a shorter duration with a better safety profile. These treatments lead to a reduction and even disappearance of cryoglobulins, as well as improvement in cutaneous vasculitis and rashes, and arthralgia and arthritis. However, there is no definite improvement in kidney functions or polyneuropathy and recurrence can occur mostly with pegylated interferon-α plus ribavirin within months after discontinuation of therapy.
Although clinical improvement can occur even without achieving HCV RNA clearance, patients who achieved sustained virologic response are more likely to have improvement in their disease manifestations.
Plasma exchange and plasmapheresis
Plasma exchange and plasmapheresis can be useful for acute and severe diseases such as rapidly progressive renal failure, severe myocarditis, central nervous system or gut vasculitis, distal gangrene or advanced neuropathy. Apheresis, however, does not treat the underlying disease and can lead to rebound increase in cryoglobulin production following cessation of therapy.
Rituximab
Rituximab (anti-CD20 monoclonal antibody) is an interesting therapeutic option for mixed cryoglobulinemia vasculitis, as it targets B cells, which are responsible for cryoglobulin production and finally, vasculitis lesions. A few recent clinical trials performed in patients with cryoglobulinemia have shown a strong benefit of adding rituximab to the standard of care with shorter mean time to clinical remission, better renal response rate and higher cryoglobulin clearance. The efficacy of rituximab in cryoglobulinemia from other etiology has also been demonstrated to be superior to immunosuppressants (i.e. azathioprine, cyclophosphamide) in terms of clinical complete remission.
Yet, rituximab should be administered with caution in mixed cryoglobulinemia vasculitis since, being itself an IgG, it may form complexes with rheumatoid factor (anti-IgG), leading to accelerated cryoprecipitation and to severe systemic reactions. This may require use of plasma exchanges prior to rituximab infusion in patients with high baseline levels of mixed cryoglobulin.
Download images for this case
Final Outcome
Two years later, Peter developed severe weakness and fever. His blood analysis showed anemia thrombocytopenia (low platelet count) and type II cryoglobulinemia (polyclonal IgG and IgM and monoclonal IgM k). Bone marrow examination showed B cell infiltration with clonal IgM k restriction, which was compatible with a diagnosis of CD5-negative non-Hodgkin lymphoma. He was finally treated with direct-acting antiviral agents for HCV infection. He was also administered rituximab (anti-CD20 monoclonal antibody) in relation to the lymphoma diagnosis.
He is now asymptomatic and has normal bone marrow.
Download images for this case
References
Bhandari, J., Awais, M. and Aeddula, N. R. (2022) “Cryoglobulinemia,” in StatPearls [Internet]. StatPearls Publishing.
Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med 1974;57:775-788
Cacoub P, Comarmond C, Domont F, Savey F, Saadoun D. Cryoglobulimemia Vasculitis. Am J Med 2015; 128:950-955
Cavallo R, Roccatello, D, Menegatti E, Naretto C, Napoli F, Baldovino S. Rituximab in cryoglobulinemic peripheral neuropathy. J Neurol 2009; 256:1076-1082
Charles ED, Dustin LB. Hepatitis C Virus-induced Cryoglobulinemia. Kidney Int 2009; 76:818–824
Dammacco F, Sansonno D. Therapy for hepatitis C virus-related cryoglobulinemic vasculitis. N Engl J Med 2013;369:1035-1045
De Vita S, Quartuccio L, Isola M, et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum 2012;64:843-853.
De Vita, S, Soldano, F, Isola, M. Preliminary classification criteria for the cryoglobulinaemic vasculitis. Ann Rheum Dis 2011;70:1183.
Muchtar E, Magen H, Gertz M A. How I treat cryoglobulinemia. Blood 2017;129:289-298
Roccatello D, Baldovino S, Rossi D, Mansouri M, Naretto C, Gennaro M, Cavallo R, Alpa M, Costanzo P, Giachino O, Mazzucco G, Sena LM (2004) Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinaemic glomerulonephritis. Nephrol Dial Transplant 2004;19:3054–3061
Sène D, Ghillani-Dalbin P, Amoura Z, Musset L, Cacoub P. Rituximab May Form a Complex With IgMK Mixed Cryoglobulin and Induce Severe Systemic Reactions in Patients With Hepatitis C Virus–Induced Vasculitis. Arthritis Rheum 2009;60:3848-3855
Terrier B, Cacoub P. Cryoglobulinemia vasculitis: an update. Curr Opin Rheumatol. 2013;25:10-18
Vannata B, Arcaini L, Zucca E. Hepatitis C virus-associated B-cell non-hodgkin’s lymphomas: what do we know? Ther Adv Hematol 2016;7:94-107
Download images for this case
Evaluation – Questions & answers
Why was HCV-associated mixed cryoglobulinemia vasculitis the final diagnosis?
Peter initially presented asthenia, inflammatory arthralgia and purpura. This is the classical triad for the diagnosis of cryoglobulimemia. Neuropathic pain of the lower limb is related to polyneuropathy. In the majority of cases, neurological involvement in HCV-associated mixed cryoglobulinemia vasculitis is secondary to a sensitive axonal symmetrical polyneuropathy.
Besides, the diagnosis of rheumatoid arthritis was not retained because of the absence of evocative polyarthritis and anti-CCP autoantibodies. Other autoimmune diseases generally start before 40 years of age and antinuclear antibodies were negative as well as anti-MPO and anti-PR3. Skin biopsy revealed typical leucocytoclastic vascularitis with no argument for IgA vasculitis or polyarteritis nodosa.
Peter presented all the clinical items of the classification criteria (constitutional symptoms, articular, vascular and neurologic involvement). He was infected with HCV, the most frequent infectious disease associated with cryoglobulinemia. Laboratory investigations showed reduced serum C4 and positive serum rheumatoid factor. Finally, he was positive for serum cryoglobulins.
What are the arguments in favour of the immune complex nature of this disease?
Do you know another disease which involves immune complex vasculitis?
Infections or drugs could lead to immune complex diseases with exogenous antigens. Examples include post-streptococcal glomerulonephritis, serum sickness, reactive arthritis, and endocarditis. In polyarteritis nodosa, the antigen may be Hepatitis B Virvus (HBV) in the same manner as HCV in cryoglobulinemia. Exogenous antigens can also be non infectious such as in farmer’s lung disease or Arthus reaction.
Which type of cryoglobulinemia is Peter affected by?
How can you explain onset of non Hodgkin lymphoma?
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz










