- Patient Presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final Outcome
- References
- Evaluation - Questions & answers
- MCQ
Patient Presentation
A 59 year old male presents to the medical out patient department with a one month history of progressively increasing fatigue and backache.
Acknowledgement
This case study was kindly provided by Dr Monica Mercer, MBChB from Immunopaedia
History
For the last month the patient has been feeling exhausted for no apparent reason and due to this extreme tiredness, is finding it difficult to work and look after himself. He comes home in the late afternoon often too tired to eat and goes straight to bed; a change from his former energetic lifestyle.
He has also been complaining of some backache which he treats with over the counter analgesics and he has been suffering from more colds and flu then usual over the last year.
He has also found that for the last 6 months there has been a decline in his ability to speak clearly. His tongue has enlarged and become firm which makes moving it for speech formation and eating solid foods difficult and at times painful.
He has had an unintentional weight loss of ± 15kg in the last 6 months.
Past medical history
Previously well, no chronic disease, no previous admissions.
Past surgical history
No history of any surgical procedures.
Family History
- Father died of a myocardial infarction at age 63 yrs with hypercholesterolaemia.
- Mother has hypertension on treatment and reports good control.
- No positive family history for any other chronic diseases including diabetes and cancer.
Social history
- Lives alone in a town house.
- Employed as an accountant.
- 30 year history of smoking.
- Significant alcohol history for 12 years, now in recovery for the last 5 years.
Differential Diagnosis
- Anaemia
- Vitamin B12/folate deficiency
- Heart failure
- Malignancy
- Multiple myeloma
- Monoclonal gammopathy of undetermined significance (MGUS)
- Waldenstrom’s macroglobulinaemia
Examination
General
- Ill looking, pale and underweight middle-aged gentleman
- Awake and alert, able to give an accurate history
- Significant dysarthria for the last 3 months, making him difficult to understand.
Vitals
- Afebrile
- Blood pressure 100/68
- Heart rate 84
- Respiratory rate 18
General
- Mild pallor
- No lymphadenopathy
- No signs of dehydration
- No jaundice
- No oedema
- No stigmata of HIV
- Tongue red and enlarged with deep imprints from the teeth on both sides of the tongue. Very reduced mobility; unable to protrude or lift up and very limited sideways movement.
Respiratory
- Trachea centrally located.
- Chest clear on auscultation.
Cardiovascular
- No raised JVP
- Normally placed apex beat
- S1 and S2 heart sounds present, no murmurs.
- No abnormalities detected
Abdomen
- Not distended
- Soft and non tender
- Bowel sounds present
- No abnormalities detected
Neurological
- Higher function intact, although difficult to understand
- Gait normal
- Power 5/5 globally
- Tone normal globally
- Reflexes 2/4 for both upper and lower limbs
- No abnormalities detected
Dermatological/Haematlogical
- Some small bruises on arms and legs of various ages
- No rashes
- No petaechial bleeds
| Examination | Value | Normal Limits | |||
|---|---|---|---|---|---|
| admission | Day 3 | Day 6 | Day 9 | ||
| WBC | 5.79 | 6.26 | 6.56 | 6.04 | (4-12 x109/L) |
| HB | 5.4 | 12.8 | 10.9 | 13 | (12.1-15.2 g/L) |
| Platelets | 150 | 129 | 97 | 130 | (140-450 x109/L) |
| CRP | 18 | 14 | (0-8mg/L) | ||
| Differential : | |||||
| Neutrophils | 8.73 | (2.00-7.5) | |||
| Monocytes | 0.41 | (0.18-0.80) | |||
| Lymphocytes | 0.57999999999999996 | (1.00-4.00) | |||
| Erythrocytes | 0.03 | (0.00-0.45) | |||
| Basophils | 0.02 | (0.00-0.20) | |||
| NA | 139 | 140 | 141 | 146 | (135-147 mmol/L) |
| K | 6 | 5.3 | 5.4 | 6.2 | (3.3-5.0 mmol/L) |
| CL | 105 | 108 | 110 | 119 | (99-103 µmol/L) |
| C02 | 18 | 16 | 18 | 11 | (18-29 mmol/L) |
| Urea | 23 | 32 | 37 | 45 | (2.5-6.4 mmol/L) |
| Creatinine | 390 | 291 | 262 | 316 | (62-115 mmol/L) |
| Total protein | 61 | 69 | 71 | (60-80g/L) | |
| Albumin | 35 | 31 | 33 | (35-50g/L) | |
| Corrected Calcium | 3.09 | 3.1 | 2.96 | 2.8 | (2.1-2.6mmol/L) |
| Phosphate | 2.41 | 1.88 | 1.87 | 1.83 | (1.0-1.5 mmol/L) |
| Magnesium | 1.1599999999999999 | 1.01 | 0.97 | 1.02 | (0.8-1.3) |
| IgG | 3.24 | (4-10) | |||
| IgM | 25.7 | (0.5-2.2) | |||
| IgA | (0.5-2.2) | ||||
| B-2 microglobulin | 22.3 | ( | |||
| HIV Elisa | negative | ||||
| Hepatitis studies, A, B and C | All negative | ||||
| Blood Culture | Negative | ||||
| Bence Jones Protein | Positive | ||||
| Bone Marrow Aspirate- phenotypic markers: | |||||
| CD38 | Increased levels detected | ||||
| CD138 | Increased levels detected | ||||
| Protein Electrophoresis | M band |
Discussion
This case study looks at a disease caused by the accumulation of neoplastic plasma B cells in bone marrow, which produce a monoclonal immunoglobulin protein found in the serum or urine. In serum, this is called an M protein or paraprotein. In urine, Bence-Jones proteins can be found which are specifically immunoglobulin light chains. We will examine the immunological basis of multiple myeloma, which is marked by upregulation of osteoclast activity due to overexpression of cytokines, causing lytic bone lesions. Multiple myeloma occurs in both men and women, but more common in men and manifests from age 40 with a peak at 60 years.
In this discussion we will look at the destructive disease process which symbolises multiple myeloma and secondary immunoglobulin light chain amyloidosis, with the aid of our full colour graphics.
Multiple myeloma
Multiple myeloma is a malignancy of B cells, specifically plasma cells, which is caused by abnormal genetic changes in B cells activated in the postgerminal centre of secondary lymphoid organs. B cells express cell-surface receptors that recognise antigens displayed on follicular dendritic cells in the germinal centres and become primed. CD4 T cell help is then required for differentiation and maturation into antibody-producing plasma cells. In individuals predisposed to multiple myeloma, DNA damage can occur following activation of B cells in the germinal centre. Specifically, abnormal DNA recombination occurs during isotype switching and affinity maturation. This results in translocation of the heavy and/or light immunoglobulin genes to other chromosomes, which influence the regulation of oncogenes or cell-proliferation genes. This primary event generates a malignant phenotype, which can be followed by secondary genetic alterations such as loss or duplication of chromosomes or genetic mutations affecting the expression of tumour-suppressor genes or oncoproteins.
Collectively, the genetic aberrations commonly found include:
- Translocation of immunoglobulin heavy chain coding regions onto other chromosomes located near protooncogenes (c-myc, n-myc and MAF) or cell- proliferation proteins (cyclin D, FGFR3, MMSET).
- Duplication of chromosomes (3, 5, 7, 9 11 and 21) or loss of chromosomes (13).
- Mutations in oncoproteins (N-ras and K-ras) or in tumour suppressor genes (p53).
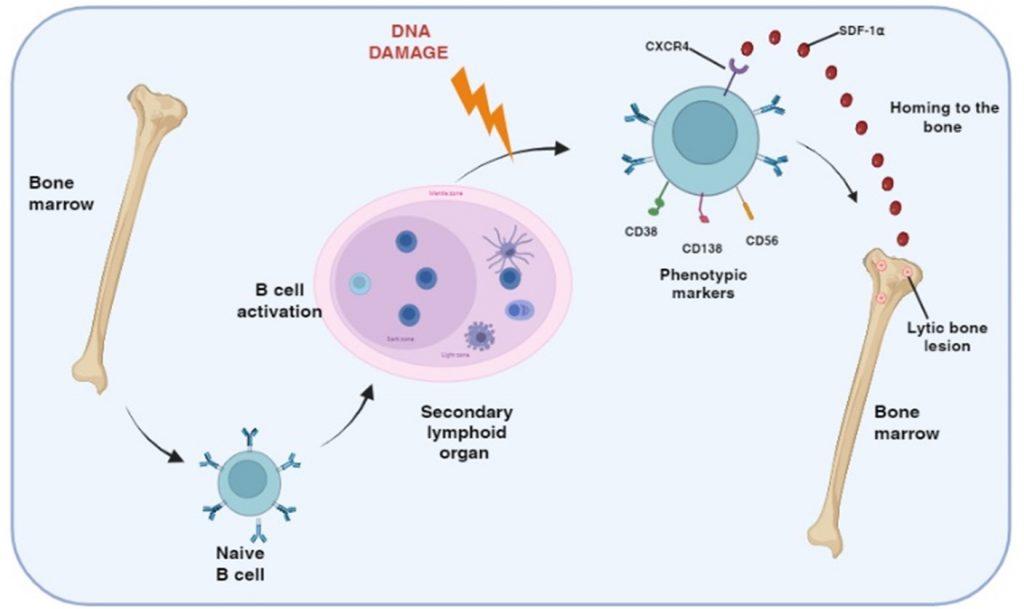
Figure 1 Generation of post-germinal center myeloma plasma B cells. Multiple myeloma is a malignancy of plasma B cells caused by alterations to genetic material following B cell activation in the germinal centers of secondary lymphoid organs. Genetic defects include primary translocation of immunoglobulin heavy/light chain genes to other chromosomes near oncogenes/cell-cycle proteins and secondary changes including duplication/loss of chromosome and/or mutations in cell growth tumor suppressor genes. Myeloma plasma B cells also express CXCR4 receptors for SDF-1α, a chemokine that regulates homing to the bone marrow where bone disease develop.
Following the maturation process and isotype switching, myeloma plasma B cells produce monoclonal antibodies, although non-secretory or light chain only forms are known. This can occur with any of the immunoglobulins, IgG being the most common, IgA and IgE occurring intermediately and IgD and IgM occurring rarely; as in this case study (IgM gammopathy).
Myeloma plasma B cells constitutively produce monoclonal immunoglobulins resulting in a gammopathy, which can cause secondary light chain amyloidosis due to deposition of excess insoluble proteins. Common sites for this include the tongue, kidneys and heart, as in this presenting case. Furthermore, circulating light chains are deposited in almost any tissue, which leads to dysfunction of the organ and subsequently the organ system. Early cardiac amyloidosis is a serious diagnostic problem. The classic signs of “right-sided” decompensated heart failure may not appear until the heart becomes neglected, which was not noted in this patient. Heart damage is the main cause of morbidity and mortality in systemic amyloidosis due to remodelling of the ventricle and low cardiac output (Novosad et al., 2023).
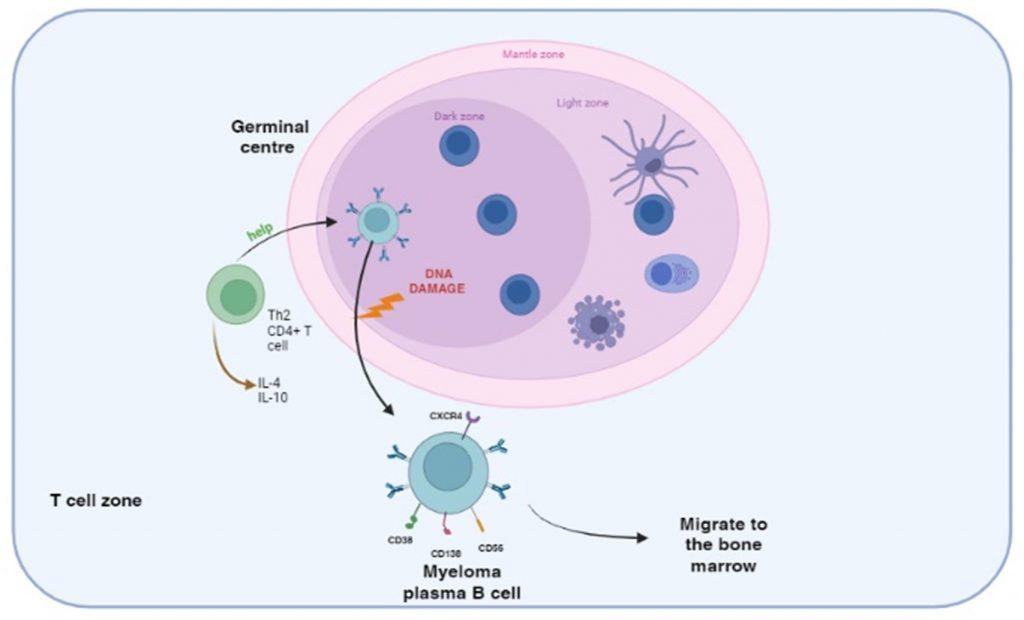
Figure 2 DNA damage following activation of B cells in the germinal centre. B cells are primed by B cell receptor recognition of antigens presented by follicular dendritic cells in the germinal centres of secondary lymphoid organs. CD4+ helper T cell interaction activates B cells to differentiate into plasma cells and also induces isotype switching and affinity maturation. During this process abnormal DNA recombination events translocate heavy and light immunoglobulin genes to other chromosomes and generate a malignant phenotype. Secondary genetic alterations involving duplication/deletion of chromosomes or mutations in other genes can follow. The myeloma plasma B cells continue to produce immunoglobulins resulting in gammopathy and ultimately home to the bone marrow where bone disease develops.
Myeloma plasma B cells migrate to the bone marrow. This homing is regulated by the chemokine stromal cell derived factor-alpha (SDF-1α) binding to the CXCR4 receptor, expressed on the myeloma plasma B cell. The myeloma plasma B cells also express cellular adhesion molecules, which interact with bone marrow stromal cell ligand. This interaction maintains myeloma B cells in an anti-apoptotic state and unresponsive to chemotherapeutic drugs.
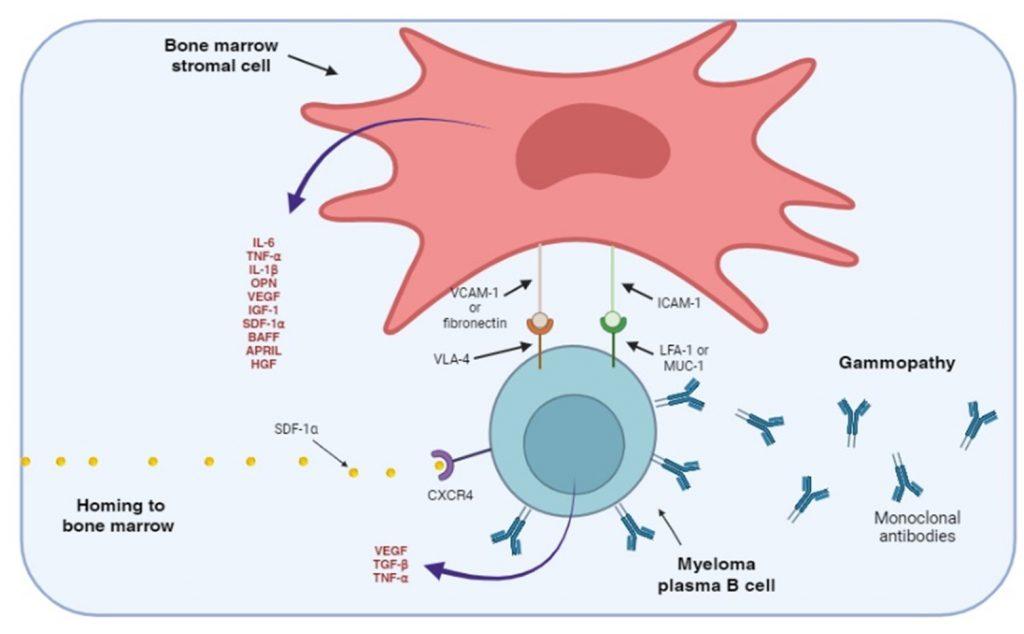
Figure 3. Adhesion of myeloma plasma B cells to bone marrow stromal cells. The myeloma plasma B cells express CXCR4 receptors that bind SDF-1 α, a chemokine that regulates homing to the bone marrow. Myeloma plasma B cells also express cellular adhesion molecules, such as VLA-4, LFA-1 and MUC-1, that interact with ligands expressed on bone marrow stromal cells. Stimulation of adhesion molecules on stromal cells generate intracellular signals that promote the secretion of soluble cellular factors that help maintain the myeloma B cells in an anti-apoptotic and drug resistant state. Soluble cellular factor that stimulates the bone marrow stromal cells are also secreted by myeloma B cells.
The accompanying bone disease is characterised by the presence of lytic bone lesions that result from an imbalance of bone formation, mediated by osteoblasts, and bone resorption, mediated by osteoclasts, which together remodel the bone microenvironment.
Bone destruction is a hallmark of the disease primarily driven by the activation of multinucleated osteoclasts. This is mediated by stimulation of RANK receptors by RANKL. In addition, the inhibitor of osteoclast activation, osteoprotegerin (OPG), is removed and degraded by CD138 which is expressed on myeloma plasma B cells, resulting in prolonged osteoclast activation.
Furthermore, bone formation by osteoblasts is inhibited by soluble factors released by myeloma plasma B cells and the secretion of Activin A from bone marrow stromal cells.
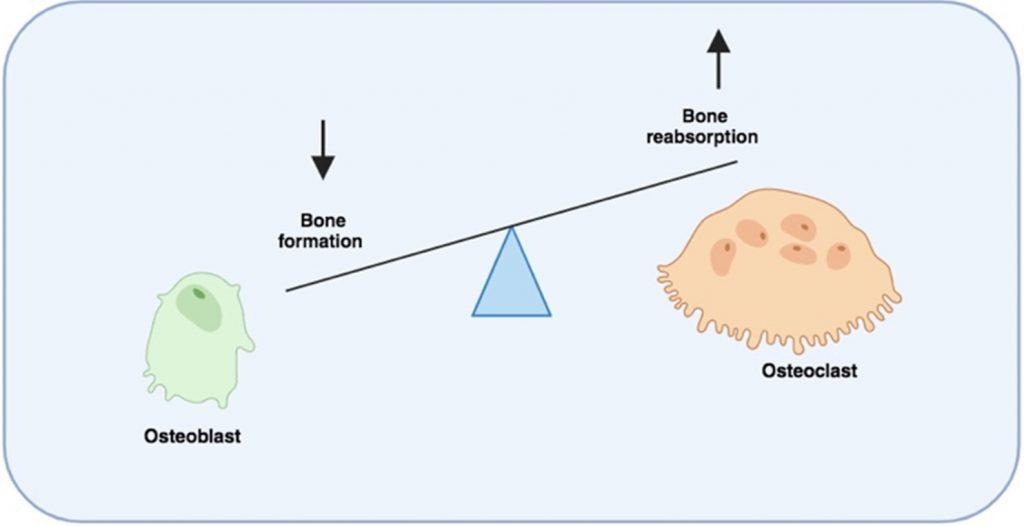
Figure 4 Bone destruction mediated by unbalanced bone formation/resorption. In multiple myeloma, bone disease is characterised by the presence of lytic bone lesions and raised blood calcium levels as a result of an imbalance between bone formation (mediated by osteoblasts) and bone resorption (mediated by osteoclast). Myeloma plasma B cells home to bone marrow and via interaction with bone marrow stromal cells introduce changes in the bone marrow microenvironment. Various soluble factors secreted by cells in the bone marrow collectively reduce bone formation and promote bone resorptions. Created in https://BioRender.com (CA Petersen 2024)
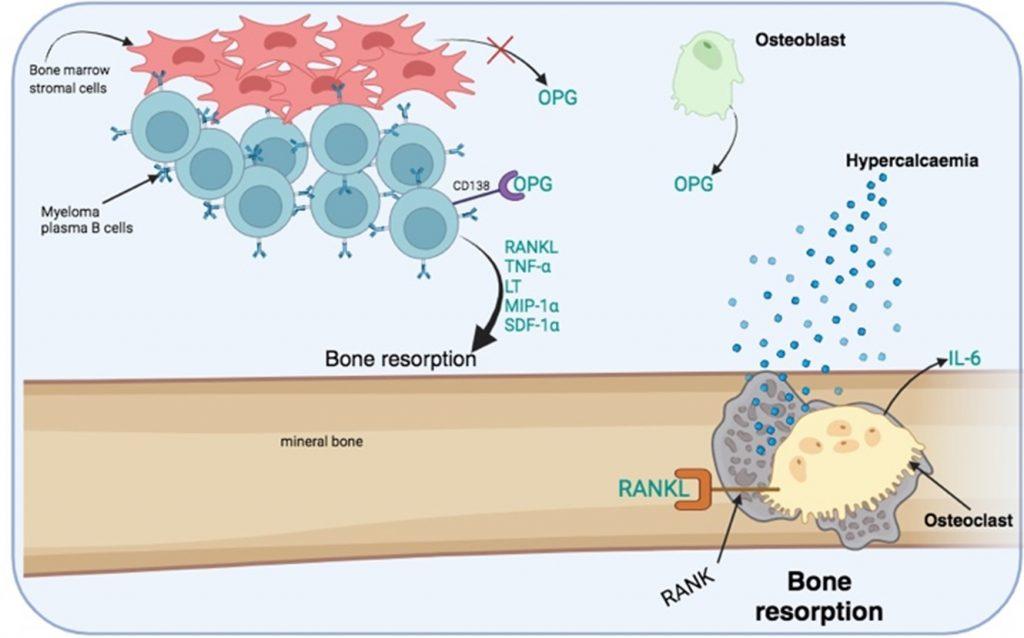
Figure 5 Increased bone resorption by osteoclast activation. Bone destruction is mediated by the bone resorption activity of multinucleated osteoclasts. RANKL is a required activation signal for osteoclasts. RANKL is secreted along with other growth factors by myeloma plasma B cells. In addition, an inhibitor of osteoclast activation, osteoprotegrin (OPG), which is secreted by osteoblast and bone marrow stem cells, is degraded by CD138 receptors expressed on myeloma plasma B cells. Bone marrow stromal cells also have reduced production of OPG. OPG acts as a decoy molecule that competes with RANKL for binding to RANK receptors expressed by osteoclasts. Activated osteoclast secrete IL-6 that promotes the survival of myeloma plasma B cells. Created in https://BioRender.com (CA Petersen 2024)
Clinical markers of multiple myeloma
The process of bone resorption creates lytic bone lesions, which liberates calcium phosphates from the mineral bone matrix. This is measurable as hypercalcaemia and hyperphosphataemia in the peripheral blood, as observed in our patient. Lytic bone lesions are visible on X-rays and most often seen on chest X-rays when examining the ribs as well as the skull (where the lesions are seen as a pepper pot skull). The increased number of plasma B cells in bone marrow can be identified from a bone marrow biopsy and are usually present at greater than 10% of the total cells: indicating a neoplastic condition. To confirm the diagnosis, multiple myeloma plasma B cells can be phenotypically characterized as CD38, CD138 and CD56 positive and negative for CD19, CD20 and CD21 expression. Genetic analysis to identify abnormal translocations, loss or duplication of chromosomes or mutations can also be used to confirm the diagnosis.
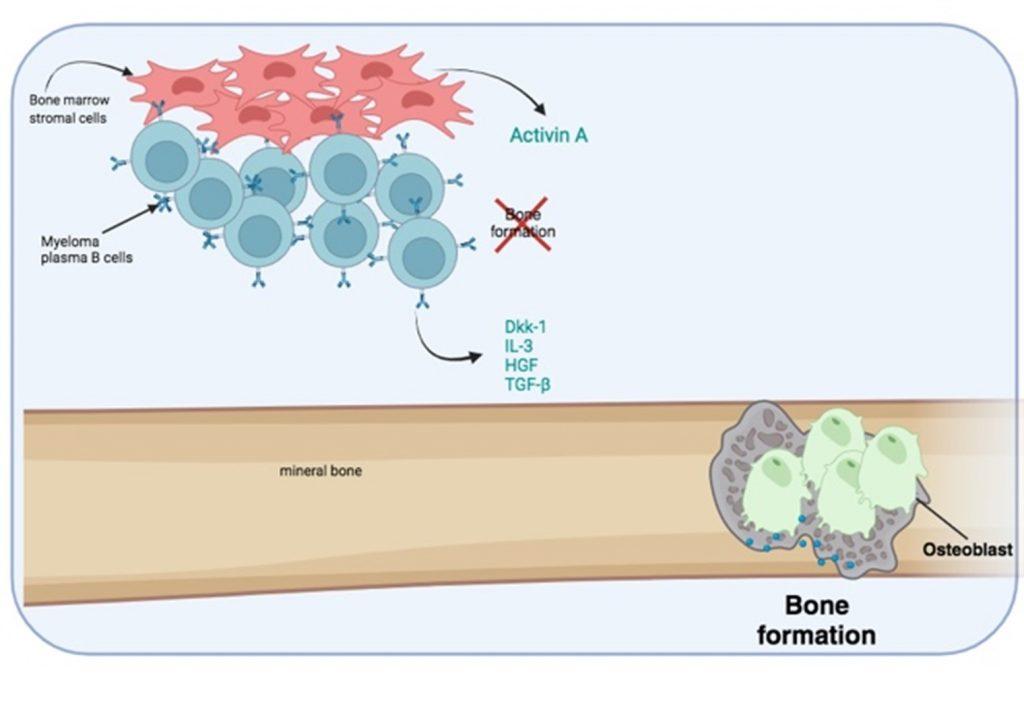
Figure 6 Bone destruction is exacerbated by the inhibition of osteoblast activity needed for bone formation. Myeloma plasma B cells secrete soluble factors that inhibit osteoblast activity. In addition, bone marrow stromal cells secrete activin A which also inhibits osteoblast function. Created in https://BioRender.com (CA Petersen 2024)
Since this is a disease of post-germinal centre B cells which implies that the B cells have isotype-switched, the gammopathy usually involves IgG (most common), IgA and IgE monoclonal antibodies and rarely IgD and IgM. In this case study, a rare IgM gammopathy indicates the absence of a class switch following post-germinal activation. We can speculate that the formation of a myeloma plasma B cell phenotype was associated with incomplete heavy chain class switching or may have resulted from aberrant light chain recombination and/or affinity maturation.
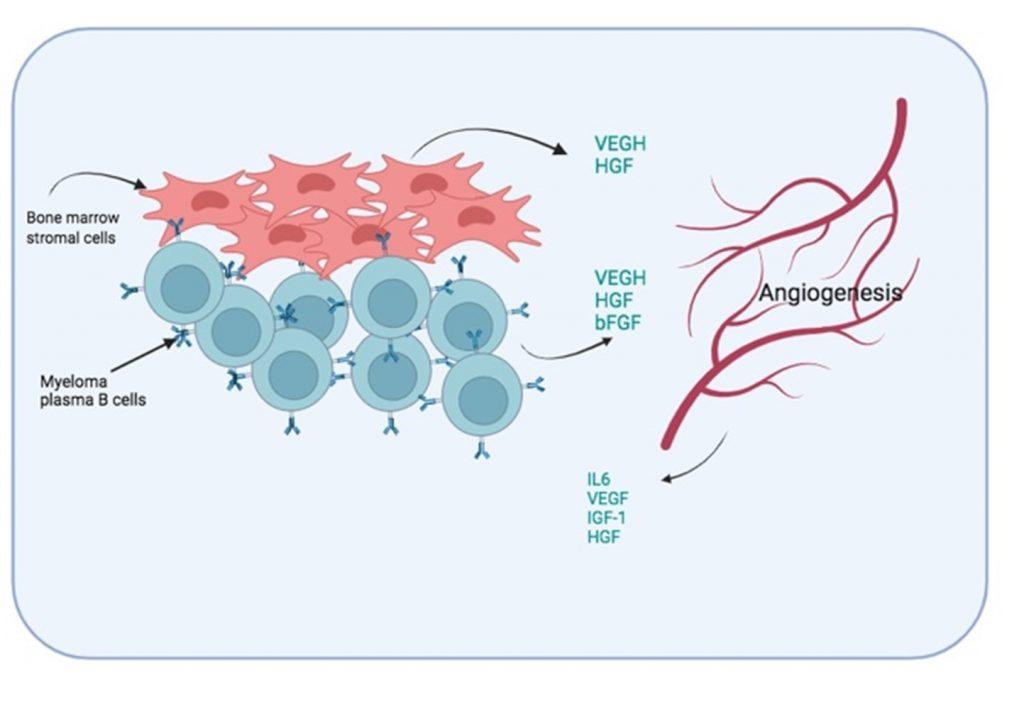
Figure 7 Induced angiogenesis in bone marrow. To improve oxygen and nutrient supply to the myeloma plasma B cells, the formation of new blood vessel is promoted by soluble factors secreted by both bone marrow stromal cells and myeloma plasma B cells. In addition, blood vessel endothelial cells also secrete growth factors that promote the survival of the myeloma plasma B cells.
The clinical manifestation in this patient was the accumulation of large quantities of antibodies and probable deposition of insoluble complexes of light chains (amyloidosis) in organs such as the tongue, kidney and heart. This was the likely cause of kidney and heart failure, the former indicated by high blood creatinine levels. Immunoglobulin light-chains were also detected in urine samples (Bence-Jones protein) and due to the high turnover of myeloma cells in bone marrow, elevated beta-2-microglobulin shedding occurs and this protein also accumulates to high levels in blood and reflects the staging of the cancer. As seen in our patient, excessive plasma levels of beta-2-microglobulin indicated highly advanced state of disease and poor prognosis. In addition, the large numbers of myeloma B cells probably displaced normal bone marrow haematopoietic cells and disrupted their function, as indicated by bicytopaenia: reduced red cell production (anaemia) and platelet formation (thrombocytopaenia). Disruption of B cell maturation in bone marrow can also impact on humoral immunity and is often related to an increased risk of infection.
When diagnosing multiple myeloma with IgM gammopathy it is important to consider other similar malignant plasma cell medicated diseases as differentials which include Waldenstrom’s macroglobulinaemia and monoclonal gammopathy of undetermined significance (MGUS). While MGUS always precedes both multiple myeloma and Waldenstrom’s macroglobulinaemia, MGUS does not always progress to bone marrow involvement. For this reason MGUS alone is not predictive of the onset of multiple myeloma. Differentiating an IgM secreting multiple myeloma from Waldenstrom’s macroglobulinaemia is based on the absence of bone disease in the latter. In our case, there was clear evidence of bone disease (X-ray and hypercalcinaemia).
Treatment
- On admission patient was transfused with three units of packed red cells to correct the anaemia.
- Zoledronic acid (Zometa) was given to control the hypercalcaemia and protect the patient from other skeletal events such as spinal cord compression and pathologic fractures.
- Patient was treated with Decadron 20 mg IV daily (dexamethasone)
- Patient was rehydrated and treated with IV fluids in an attempt to improve her renal function before administration of chemotherapy.
- Plan was to give patient chemotherapy- VAD (vincristine, doxorubicin [Adriamycin], and dexamethasone) to decrease the tumour burden in multiple myeloma. This is typically used in preparation for autologous stem cell transplantation. VAD is administered as a 4-day continuous intravenous infusion of vincristine and doxorubicin, with 4 daily oral doses of dexamethasone. Patients require a central venous catheter for delivery of the infusion. In selected patients, this therapy can be performed in an outpatient setting.
- Although not administered in this patient Thalidomide is a treatment of choice for multiple myeloma patients.
Effectiveness of treatment for multiple myeloma depend significantly on timely diagnosis. Over the past three decades treatment has improved significantly based on the use of targeted drugs in combination with or without chemotherapy, followed by consolidating therapy and autologous haemopoietic stem cell transplantation (Novosad et al., 2023).
Monoclonal antibodies show great promise like with daratumumab, inducing a rapid response against plasma cells specifically targeting CD38, a transmembrane glycoprotein over expressed on the surface of plasma cell clones on multiple myeloma and AL amyloidosis, and expressed at low levels on hematopoietic as well as non-hematopoietic cells (Zhang & Comenzo, 2022).
Final Outcome
The patient developed renal failure, which remained refractory to treatment. He also developed a cardiac myopathy due to cardiac amyloidosis and died two days later from cardiac failure.
References
Vallet S et al. (2010). “Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease.” Proceedings of National Academy of Science USA, 1 March
Link to Abstract
Edwards CM et al. (2008). “The pathogenesis of the bone disease of multiple myeloma.” Bone Jun:42(6):1007-13
Link to Abstract
Roodman GD. (2009). “Pathogenesis of myeloma bone disease.” Leukemia Mar:23(3):435-411
Hideshima T et al. (2007). “Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets.” Nat Rev Cancer 7(8):585-98
Evaluation – Questions & answers
What is the diagnosis?
What is the cause of macroglossia in this patient?
What else is linked to the above condition?
Which cell-type becomes neoplastic and is implicated in multiple myeloma?
What types of genetic aberrations occur in multiple myeloma?
- translocation of immunoglobulin heavy chain coding regions onto other chromosomes located near oncogenes or cell-proliferation proteins
- duplication or loss of chromosomes
- mutations in oncogenes or in tumour-suppressor genes.
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz










