- Patient Presentation
- History
- Differential diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Prevention
- Final Outcome
- MCQs
- Thought Questions
- References
Patient presentation
A previously healthy 35-year-old male presents with sudden onset of fever, fatigue, myalgia, and headache. He reports recent travel to an Ebola-endemic region in West Africa.
This case study was created by Immunopaedia Ambassador Vanessa Amana Bokagne from Eberhard Karl University Tuebingen, Germany and Centre de Recherches Médicales de Lambaréné, Gabon
History
- The patient describes close contact with a deceased relative who had similar symptoms.
- He states he has no other significant medical history.
Differential Diagnosis
- Malaria
- Typhoid fever
- Lassa fever
- Other viral haemorrhagic fevers
- Ebola haemorrhagic fever (EHF)
Examination
General
- The patient appears ill
- Generalized weakness
- Conjunctival injection
- Haemorrhagic manifestations (petechiae, ecchymoses) may be present.
Vitals
- Heart rate (beat/min): 83/min
- Blood pressure: 114/72 mmHg
- Temperature: >38°C
- Oxygen saturation: 97%
Investigations
Hyponatremia (sodium < 135 mmol/litre)
Hypocalcaemia (total calcium < 8 mmol/ litre)
Hypokalaemia (potassium < 3.5 mmol/litre)
Hypomagnesemia (magnesium < 0.85 mmol/litre)
Hypoalbuminemia (albumin < 3.5 mmol/litre)
Elevated creatinine (>1.3 mg/dl)
Elevated bilirubin (>1.5 mg/dl)
Elevated aspartate aminotransferase (>98 U mg/litre)
Elevated alanine aminotransferase (>110 U mg/litre)
Leukopenia (white cell count <3500/ul)
Neutropenia (absolute neutrophil count < 1500/ul)
Lymphopenia (absolute lymphopenia count < 1500/ul)
Platelet Anaemia (haemoglobin < 11 mg/dl)
Thrombocytopenia (platelet count < 150000/ul
Reverse transcription-polymerase chain reaction (RT-PCR)
| Diagnostics | Test | Value |
|---|---|---|
| Ebola | Ebola RT-PCR | positive |
| EBOV RNA, log10 copies/mL | 7,19 | |
| Ct value | 26,26 |
Discussion
The patient presented with signs and symptoms typical of haemorrhagic fever disease. The initial assessment of the patient raised concerns about Ebola haemorrhagic fever (EHF). This hinges on two main factors: epidemiological risk (he has been to an Ebola endemic area recently) and the presence of clinical signs such as high-grade fever (> 38.0 °C), fatigue, conjunctival injection, and haemorrhagic manifestations (petechiae, ecchymoses). Moreover, laboratory analysis of CBC showed leukopenia and thrombocytopenia which are common signs associated with EHF. Viral RNA amplification from the patient’s blood via RT PCR assay showed positive for Ebola virus. The most likely source of infection would be close contact with a deceased relative who had similar symptoms.
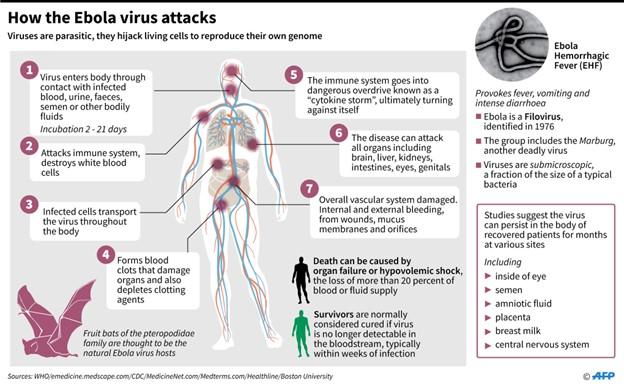
Ebola virus disease (EVD) also called Ebola haemorrhagic fever (EHF), is a zoonotic viral disease affecting both humans and other primates. The virus has been reported to have caused multiple outbreaks in West and Central Africa with case fatality rates often approaching 90 percent. It is transmitted via direct contact with bodily fluids, such as the blood of an infected person or animal, or by coming into touch with objects that have recently encountered contaminated bodily fluids. This emphasizes the precautions to take to prevent any potential transmission or contamination when a case is suspected.
EHF: clinical spectrum
Symptoms of Ebola usually appear 8–10 days after contact, with fever striking suddenly in most cases. The most typical symptoms are chills, myalgia, weariness, and raised body temperature, often known as subjective fever (typically greater than 38.0 °C).
Usually, gastrointestinal symptoms like nausea, vomiting, and diarrhoea start to show up a few days following these initial signs. There could be fluid loss of up to 5–10 L/day during this period, which can range from mild to severe. Conjunctival injection, hiccups, cough and dyspnoea, and localized pain (in the chest, belly, muscles, or joints) are some of the less common symptoms and indicators. While some patients will begin to recover at this point (sometimes following a quite mild illness), a systemic inflammatory response and hypovolemia may cause others to fall into shock. It is common for patients to appear at this time with haemorrhagic episodes, such as petechiae, gastrointestinal bleeding, mucosal haemorrhage, ecchymosis, or persistent seeping from the venepuncture site, as is the case for this patient. The patient’s haemorrhaging often happens when the virus infects the liver, impairing the body’s production of blood clot proteins and leading to blood vessel leakage.
Host immune response to EHF
Once within the host, EBOV subverts the host immune system by suppressing dendritic cell (DC) activity, antagonistic interactions with interferons, and lymphocyte depletion, including natural killer (NK) cells. The most significant early and preferred target cells by EBOV infection have been demonstrated to be monocytes, macrophages, and DCs. By focusing on these immune cells, the virus can worsen the immune response by preventing and limiting their ability to function, which also helps the infection to spread.
DCs are among the most potent antigen-presenting cells and secrete vital interleukins and cytokines that bridge the gap between innate and adaptive immune responses to a variety of pathogens. However, the vital functions of DCs infected with the Ebola virus are severely compromised. These cells are unable to develop into mature or activated cells that can stimulate T cells by upregulating major histocompatibility complex (MHC) molecules. Furthermore, the virus does not enter lymphocytes but can show interaction with CD4+ T-cells. Despite non-entry into lymphocytes, bystander lymphocyte apoptosis is observed during infection which leads to T-cell exhaustion.
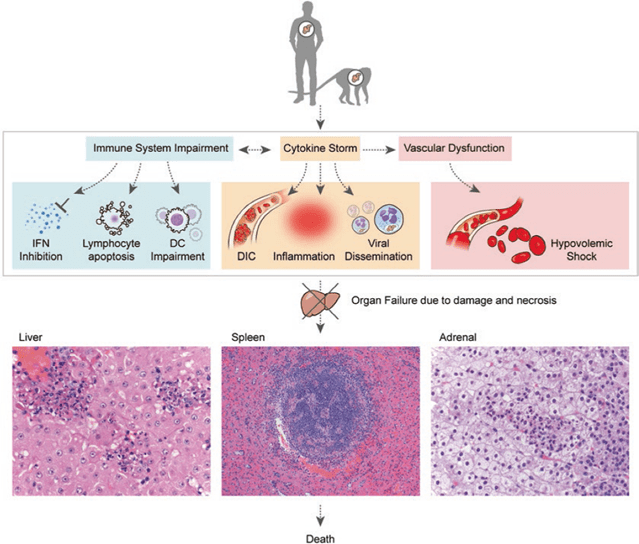
Unlike DC infection, monocyte/macrophage infection causes activation and massive cytokine production, known as the “cytokine storm,” which may also contribute to a deadly outcome. High levels of pro-inflammatory cytokines are secreted by infected macrophages, which in turn stimulate the expression of cytokines and chemokines by both infected and noninfected macrophages. This promotes a pro-coagulant state and fluid leakage into the interstitium, resulting in hypovolemic shock and the development of disseminated intravascular coagulation (DIC).
Hypovolemic shock and multiorgan failure usually result in death 6–16 days after symptoms first appear. Effective viral clearance and survival are linked to an early antibody response, mild and measurable anti-inflammatory responses during the early stages of symptoms, and the growth of a strong T cell response.
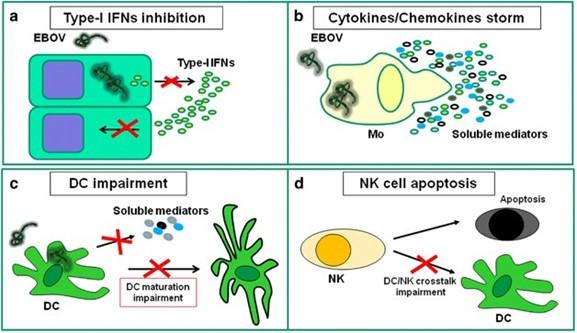
Fig 2: EBOV infection induces innate immune cell dysfunction. (a) EBOV infection is able to impair type-I IFNs production by infected cells and to block IFN response in uninfected cells; (b) EBOV infection is able to induce massive cytokines/chemokines production by monocytes/macrophages; (c) EBOV infection is able to impair DC maturation and to deregulate cytokine production. (d) EBOV infection is able to induce massive NK apoptosis, thus avoiding NK function and impairing NK-mediated DC maturation help (Goeijenbier et al., 2014)
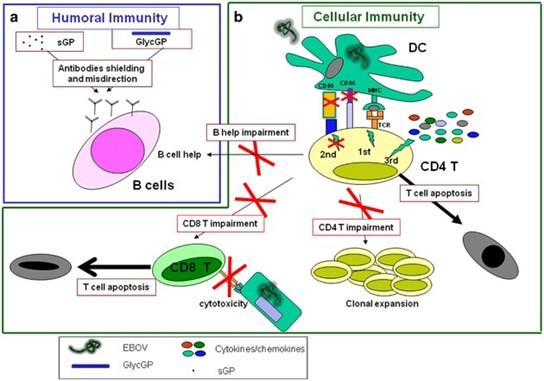
Fig 3: EBOV infection induces adaptive immune cell dysfunctions. (a) Antibodies production represents the best correlate of protection during EBOV infection. Two different forms of EBOV GP, soluble GP (sGP) and glycosylated-GP (GlycGP), are able to drive antibodies shielding and misdirection. (b) EBOV infection of DC results in a deregulated DC/T synapse, characterized by an effective MHC-peptide/TCR interaction (signal 1), in a high inflammatory microenvironment (deregulated signal 3) in the absence of co-stimulatory accessories molecules on DC surface (ineffective signal 2). The inappropriate DC/T-cell interaction induces T-cell apoptosis, avoids CD4 T-cell clonal expansion, thus blocking all CD4 T-cell helper functions such as CD8-mediated cytotoxicity and antibodies-production by B cells (Goeijenbier et al., 2014).
Pathologic events leading to fatal outcomes.
EVD is associated with several abnormalities that can be considered as possible predictors of fatal outcomes. These can be grouped into those related to haematology, clinical chemistry, and coagulation.
Haematological abnormalities include early leukopenia (as low as 1000 cells per µL) with lymphopenia and subsequent neutrophilia, left shift with atypical lymphocytes, while clinical chemistry abnormalities include elevated liver enzymes (ALT, AST, ALP, and/or GGT), decreased serum albumin, and increased serum creatinine and blood urea nitrogen (BUN). Coagulation abnormalities, which are thought to initiate the progression of disseminated intravascular coagulation (DIC), include early elevation of fibrinogen levels followed by thrombocytopenia (50000–100000 cells per µL), as well as prolonged clotting times.
The time course between the appearance of primary signs and symptoms and the severe manifestation of the disease is relatively low in fatal cases (usually around 8 days). This explains why the patient succumbs to the disease within a week.
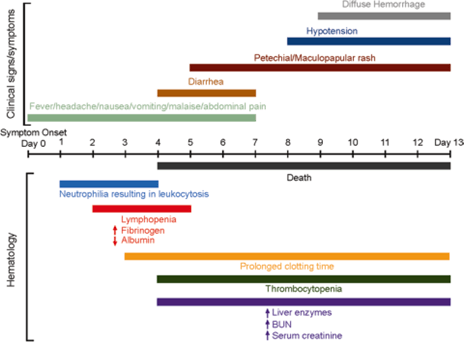
Fig 4: Gantt chart depicting clinical and haematological progression of EHF beginning at symptom onset (day 0). Clinical signs and symptoms were derived primarily from human data available after the most recent outbreak in West Africa. Haematology data were derived primarily from NHP data, as available. Human data were complicated due to treatment regimens; however, an attempt was made to correlate recent human data obtained in the West African outbreak with available NHP data (Vine et al., 2017.)
Thrombocytopenia in critically ill patients (TCIP)
Thrombocytopenia is a constant feature of EHF. It’s a condition in which a person has a low number of platelets, the blood cells responsible for clotting. To stop wounds from bleeding, platelets aid in the formation of clots or plugs. Severe thrombocytopenia patients may bleed readily and find it difficult to stop. There are various causes for thrombocytopenia. There are situations where either not enough platelets are produced by the bone marrow or the immune system, spleen, or liver removes platelets from the circulation. In the case of EHF, thrombocytopenia is mostly caused by loss of platelets from damaged tissue or more generalized virus-induced disseminated intravascular coagulation, where coagulation factors are depleted. Disseminated intravascular coagulation, along with acute hepatic impairment, predisposes the patient to bleeding complications. Acute kidney damage, hepatitis, and pancreatitis are other complications of severe illness.
Treatment
Since there are limited treatment options for Ebola virus, the patient was given experimental drugs to fight the disease, and supportive care to alleviate the numerous symptoms as recommended by WHO.
Recommended treatment
So far two drugs are being recommended by WHO (WHO,2024):
- mAb114 (Ansuvimab; Ebanga): a single human monoclonal antibody derived from an Ebola survivor.
- REGN-EB3 (Inmazeb): a co-formulated mixture of three IgG1 monoclonal antibodies
There is also a recommendation on therapeutics that should not be used to treat patients: these include ZMapp and remdesivir especially for patients with RT-PCR confirmed EHF.
However, all these recommendations only apply to Ebola virus disease caused by Ebola virus (EBOV; Zaire ebolavirus).
Adjunct therapies
Supportive care and treatment of complications are the cornerstones of the treatment of EBOV. This includes maintaining hydration, blood pressure monitoring, and nutritional support as well as symptomatic relief.
Treatment of hypovolaemia from dehydration/volume loss
Glucose 13.5 g/l, sodium chloride 2.6 g/l, potassium chloride 1.5 g/l, trisodium citrate dihydrate 2.9 g/l, (total osmolarity of 245 mOsm/l) or alternate between IV 0.9% saline and Ringer’s lactate in case the patient is not able to drink.
Fluid management in sepsis and shock algorithm
Isotonic crystalloid fluid for fluid resuscitation; 0.9% saline or Ringer’s lactate (RL) solution. Bolus with 500 ml up to 30 ml/kg or until normalization of signs of perfusion. If shock persists, despite fluid loading, vasopressors were used to maintain perfusion. In this case noradrenaline is the first-line vasopressor for shock
Fever
Paracetamol: 1 g paracetamol PO/IV every 6–8 hours. Maximum dose 4 g every 24 hours.
Pain control
Mild pain – paracetamol: 1 g PO/IV every 6–8 hours as needed, maximum dose 4 g every 24 hours. Severe pain – tramadol: 50–100 mg PO/IV every 4–6 hours as needed, daily maximum 400 mg/day. Non-steroidal anti-inflammatory drugs (including aspirin) should be avoided because of the associated increased risk of bleeding and potential for nephrotoxicity.
Haemorrhage
Transfusion if active bleeding and haemodynamic instability or Hb < 7 g/dl. Transfuse with red blood cells to target Hb > 7g/dl, fresh plasma to target international normalized ratio < 1.5, and platelets to target > 50.
High dose proton pump inhibitor for GI bleed: pantoprazole 80 mg IV over 60 minutes then 8 mg/hour for 72 hours as continuous infusion or 40 mg
Hypoxic respiratory failure
Face mask with reservoir bag was used. It was titrated to the lowest flow rate necessary to reach target SpO2 > 94%.
Treatment of potential co-infections
Co-infection with malaria: artesunate-amodiaquine (10) or pyronaridine-artesunate
Bacterial co-infection: Severe disease (IV ceftriaxone 2 g once daily 5 days +/- IV metronidazole 500 mg three times/day (usually 7 days). Mild disease (PO cefixime 200 mg twice daily for 5 days).
Prevention
Possible prevention strategy
There are 2 licensed Ebola vaccines to date. EVERBO and a second new vaccine delivered in 2 doses called Zabdeno (Ad26.ZEBOV) and Mvabea (MVA-BN-Filo) for individuals 1 year and older. The Ervebo vaccine has been shown to be effective in protecting people from the species Zaire ebolavirus and is recommended by the Strategic Advisory Group of Experts on Immunization as part of a broader set of Ebola outbreak response tools (WHO, 2024)
Other prevention strategies include:
- Washing hands
- Avoid touching the body fluids of people who have, or may have, Ebola.
- Not touching the bodies of people who have died from Ebola.
- Getting the Ebola vaccine if they are at risk for the Zaire type of Ebola.
Reduced human transmission of the Ebola virus can be achieved through increasing public knowledge of risk factors and preventive interventions (vaccination included). Messages about risk reduction should emphasize the following: Reducing the risk of wildlife-to-human transmission
- Reducing the risk of human-to-human transmission
- Outbreak containment measures, including safe and dignified burial of the dead.
- Reducing the risk of possible sexual transmission
- Reducing the risk of transmission from pregnancy-related fluids and tissue.
Final Outcome
The patient diagnosis revealed a viral infection caused by Ebola virus. He was administered regiment therapy and supportive care as recommended by WHO. However, his condition kept on deteriorating as he progressed towards a severe stage of the disease. The more severe the symptoms, the less the treatment was effective. There could be a possibility that he failed to mount an early antibody immune response against the virus, which led to rapid progression of the disease. He develops multiorgan failure, disseminated intravascular coagulation (DIC), and shock which as previously mentioned, are features of fatal outcome of EVD. Unfortunately, he succumbs to EVD within a week of admission.
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Thought Questions
- What are the main immune target cells of EBOV?
- What are survival correlates in EHF?
- What are the markers of fatal outcome in EHF?
- How can EVD be prevented?
- What are the precautions to take in case of suspicions of EHF case?
- Can you propose a pathway which could represent potential therapeutic strategy against EBOV infection?
References
- Goeijenbier M, van Kampen JJ, Reusken CB, Koopmans MP, van Gorp EC. Ebola virus disease: a review on epidemiology, symptoms, treatment, and pathogenesis. Neth J Med 2014; 72: 442–448.
- Jain S, Khaiboullina SF, Baranwal M. Immunological Perspective for Ebola Virus Infection and Various Treatment Measures Taken to Fight the Disease. Pathogens. 2020 Oct 17;9(10):850. doi: 10.3390/pathogens9100850. PMID: 33080902; PMCID: PMC7603231.
- Vine V, Scott DP, Feldmann H. Ebolavirus: An Overview of Molecular and Clinical Pathogenesis. Methods Mol Biol. 2017; 1628:39-50. doi: 10.1007/978-1-4939-7116-9_3. PMID: 28573609; PMCID: PMC11060604.
- https://www.who.int/health-topics/ebola, WHO (2024), Retrieved July 2024