- Patient presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Final outcome
- Evaluation - Questions & answers
- MCQ
- References
Patient presentation
A four and a half year old boy presents with pyrexia, neck stiffness and a purpuric rash.
Acknowledgement
This case study was kindly provided by Dr Monika Esser MMed Paed, Head of Division of Immunology, N.H.L.S Coastal Branch, Tygerberg Hospital.
History
- At age three years he had his first episode of meningococcal meningitis, from which he made a full recovery. At the time of contracting meningococcal meningitis he was on antibiotics for an upper respiratory infection he had contracted two weeks previously. Therefore no organisms were isolated from the CSF. There were no known contacts for meningitis at time of infection.
- At age three years and four months he suffered from another episode of bacterial meningitis. No culture was requested.
- At age four years he was diagnosed with meningococcal septicaemia. He made a full recovery with no sequelae.
- The infections were described as relatively ‘mild’ by the referring doctor.
- Other than the above mentioned infections this patient has developed consistently and well and is growing on the 50th centile for weight and height.
- All routine childhood vaccinations were given.
- His family history, including that of his two older female siblings is normal.
- The patient lives at home with his parents and two siblings in a 2 bedroom house with running water and electricity.
Differential Diagnosis
- Meningococcal meningitis
- Meningococcal septicaemia
- Base of Skull (BOS) fracture with cerebral spinal fluid leakage.
- Complement Deficiency
- Selective Antibody Deficiency
Examination
- Axillary temperature, 38.5 ˚C
- Purpuric rash with petechiae noted on the trunk and legs
- Neck stiffness elicited both Kernig and Brudzinski signs
- Generally patient is lethargic
Investigations
| rine dipstick | Normal |
|---|---|
| Full blood count | WBC 21 000 with (L) shift |
| Hb and platelets – normal | |
| Blood Cultures | MCS – N. meningitides |
| Lumbar Puncture | Contraindicated |
| Serum Immunoglobulins | IgG – normal |
| IgM – normal | |
| IgA – normal | |
| Pneumococcal antibody | Present, protective |
| Peticheal Smear | Gram negative diplococci (see slide) |
| Total Serum Complement | Less than 25% (Normal 80-100%) |
| Complement Fraction | Complement levels: |
| C1q – present | |
| C1r – present | |
| C1s – present | |
| C2 – present | |
| C3 – 92mg/dl (N) | |
| C4 – 25mg/dl (N) | |
| C5 – present | |
| C6 – absent | |
| C7 – present | |
| C8 – present | |
| C9 – present | |
| Results show that the total complement activity is abnormally low. | |
| Gel precipitation | Shows an absence of C6 precipitation |
Discussion
The complement System
The complement system plays a crucial role in both innate and adaptive immunity, augmenting the function of antibodies and phagocytes. Antigen-antibody immune complexes, lectin binding and accelerated C3 tick-over [the passive hydrolysis of C3 in the blood, forming the convertase with factor B] is the initiating step for this well coordinated process. The importance of the complement system is highlighted by disease that arise due to deficiencies in one of the components.
The complement pathways can be broken down into 3, of which each is initiated differently. The initial steps in the complement cascade are triggered by:
- antigen-antibody complex [classical pathway],
- lectin binding [lectin pathway],
- accelerated C3 tick-over [alternative pathway]
After initiation, protease mediated cleavage leads to activation of complement mediated functions and downstream activation of the adaptive immune response (Ling & Murali, 2019).
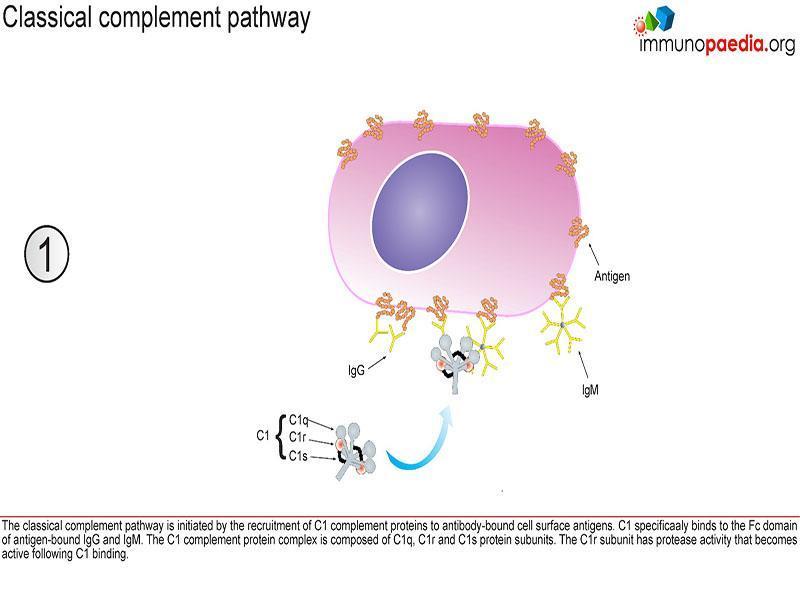
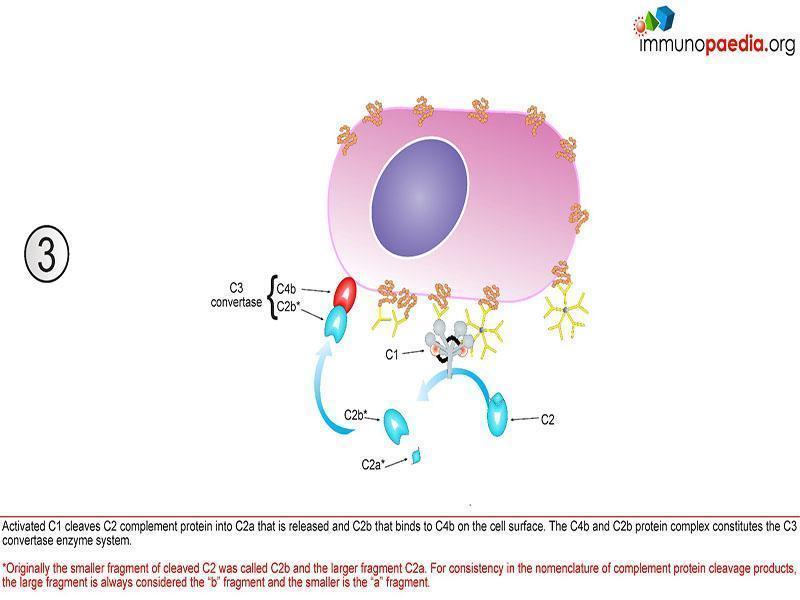
Classical pathway
This pathway is initiated by C1q that recognizes antigen-antibody complexes or pentraxins [serum pattern recognition proteins]. A conformational change then takes place allowing C1q to interact with C1r and C1s, forming a protease enzyme C1qr2s2. C1qr2s2 proceeds to cleave C4 and C2 to generate the classical pathway C3 convertase; C4b2a
C4b2a is then able to induce proteolytic activity and activate the common terminal pathway (figure 1).
Lectin pathway
The lectin pathway is triggered by circulating pattern recognition receptors (PRR); capable of recognising carbohydrates on the surface of microbes. These are mannose binding lectins (MBL) and ficolin. MLB then complexes with MLB associated serine proteases (MASP-1 and MASP-2); which cleaves C4 and C2 to form the lectin pathway C3 convertase, C4b2a (figure1) (Ling & Murali, 2019).
Alternative Pathway
The alternative pathway is activated by the aggregation of C3 tick-over, but does not proceed further if C3b does not encounter a stabilising counterpart, Bb. Bb arises a product of factor D mediated cleavage if factor B. The alternative pathway C3 convertase arises by the interaction of C3b and Bb, C3bBb. C3b is also able to covalently and stably bind to amino or hydroxyl groups on the cell surface of neighbouring pathogens allowing for complement activation on the surface of targeted pathogens. The alternative pathway can also be activated by C3b generated from the classical and lectin pathway (figure 1).
Common terminal pathway
The C3 convertase [C4b2a or C3bBb] produced via the lectin; classical and alternative pathway causes the complement cascade to proceed due to the proteolytic activity against C3. C3 is cleaved into C3b and C3a; where C3a is an inflammatory mediator and C3b complexes with C3 convertase to form C5 convertase. C5 is cleaved into C5b; which initiates the formation of the cytolytic membrane attack complex (MAC); by C5b complexing with C6; C7; C8 and C9 (figure 1) (Ling & Murali, 2019).
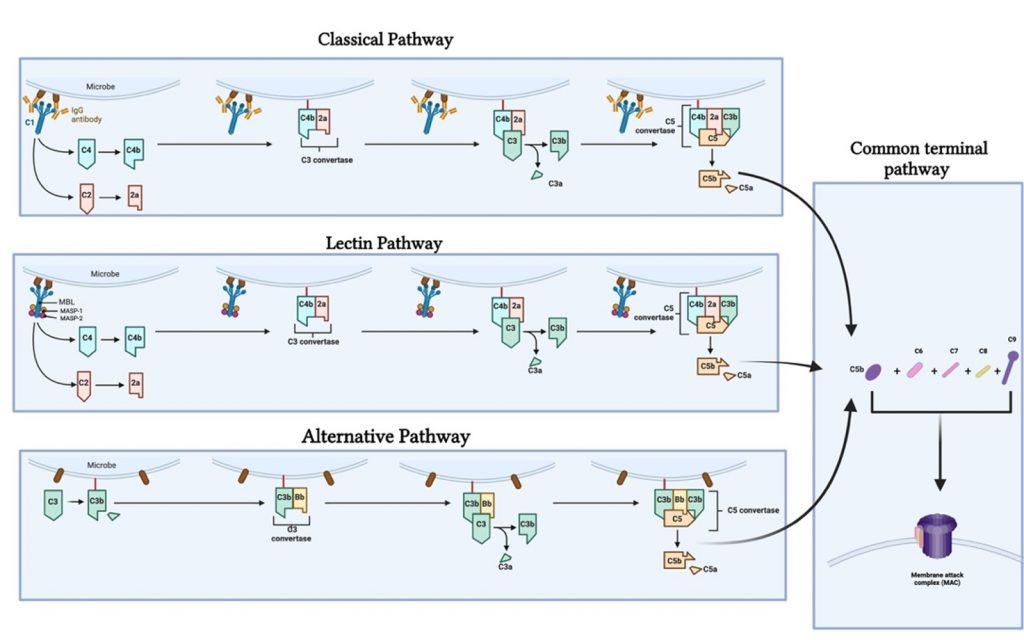
Figure 1. Illustrates the different complement pathways (classical, alternative and lectin pathway) and how they contribute to the complement cascade ending in the formation of a membrane attack complex. Created with BioRender.com (CA Petersen 2024)
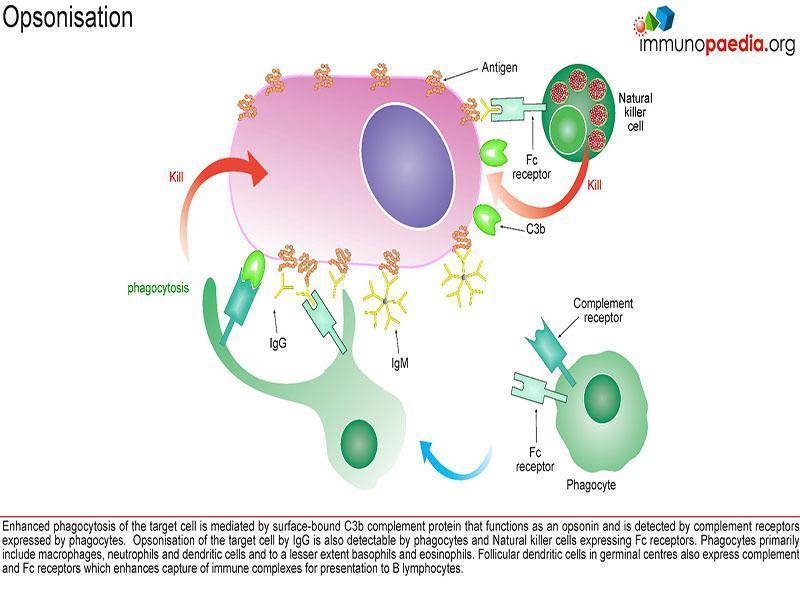
This case describes deficiencies in complement exposing patients to infection via two mechanisms:
- ineffective opsonisation 2.
- defects in lytic activity (defects in MAC)
The 3 complement pathways (Classical, Alternative and Mannose-Binding Lectin) converge at the component C3. Although each pathway is triggered differently, the common goal is to deposit clusters of C3b on a target.
This deposition provides for the assembly of the membrane attack complex (MAC), components C5b-9.
The MAC exerts powerful killing activity by creating perforations in cellular membranes.
Individuals with complement deficiencies that hinder Opsonisation have C3 deficiency and commonly get recurrent infections of S. pneumoniae.
Deficiencies of early classical pathway components (C1, C4, C2) do not usually predispose individuals to severe infections but are associated with autoimmune disorders, especially SLE and recurrent upper respiratory tract infections.
Patients with a defect in formation of the MAC (late complement components) are at high risk for recurrent infection with Neisseria gonorrhoeae or Neisseria meningitidis (Ling & Murali, 2019; Moya-Quiles et al., 2013).
Download images for this case
Treatment
- Empirically dosed systemic Penicillin for current infection.
- Prophylaxis with oral or IM Penicillin for life.
Download images for this case
Final outcome
Currently he is well and exhibiting a good response to Penicillin treatment.
Download images for this case
Evaluation – Questions & answers
Explain why you would request Complement Function tests in the light of the possible Immunodeficiencies that you are expecting.
If low activity is documented this indicates deficiency in complement factors. Specifically deficiency of C6, 7, 8, 9 indicates a susceptibility to Neisseria organisms.
What is the role of complement in the normal function of the immune system?
Complement consists of cell surface proteins and a system of serum proteins which interact with one another as well as with other molecules of the immune system. These affect both the innate and adaptive immune responses. The three pathways of activation depend on the initiating stimulus:
- Classical complement pathway is activated by antigen-antibody complexes
- Alternative pathway by microbial surfaces
- Lectin pathway by plasma lectins that bind to microbes
Each of the pathways consist of a cascade of inflammatory mediators and opsonin’s and leads to a lytic complex that inserts into the cell membrane.
Why is the patient not unusually susceptible to other microorganisms ?
The patient has normal opsonization for bacteria because of intact activation via complement C3. The terminal complement components C5-C9 form the membrane attack complex (MAC). This serum bactericidal activity is crucial in the defence against Neisseria species, but interestingly not to a variety of other gram-negative organisms which require bactericidal activity. So, with this deficiency it is only Neisseria species that the patient is susceptible to.
What are guidelines to management of this patient to prevent recurrences of these infections?
- Prophylactic Penicillin daily PO (Pen K) or IMI 3 weekly (Bicillin)
- Boosting of immune response with meningococcal vaccine
- Screen for and treat carriers in the house
- Screen family for Complement deficiency
- Medical Alert Badge
Why would you request a Pneumococcal antibody test even though the Serum Immunoglobulins (SeIg) are normal?
Significant Functional Antibody Deficiencies can occur despite normal SeIg levels. These can be selective for specific organisms particularly for the Pneumococcal infections. The first two episodes of recurrent meningitis had no culture isolate and therefore cannot be assumed to have been due to N. meningitidis.
If the patient had repeated low or absent SeIg of the IgG class, which organ systems would you expect to be involved with recurrent infections?
Upper respiratory tract – sinusitis, otitis media
Lower respiratory tract – pneumonia, empyema
GIT – diarrhoea and particular susceptibility to Giardia lamblia infection
General – Failure to Thrive
Download images for this case
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
Download images for this case
References
Ling, M., & Murali, M. (2019). Analysis of the Complement System in the Clinical Immunology Laboratory. Clinics in Laboratory Medicine, 39(4), 579–590. https://doi.org/10.1016/J.CLL.2019.07.006
Moya-Quiles, M. R., Bernardo-Pisa, M. V., Martínez, P., Gimeno, L., Bosch, A., Salgado, G., Martínez-Banaclocha, H., Eguia, J., Campillo, J. A., Muro, M., Vidal-Bugallo, J. B., Álvarez-López, M. R., & García-Alonso, A. M. (2013). Complement component C6 deficiency in a Spanish family: implications for clinical and molecular diagnosis. Gene, 521(1), 204–206. https://doi.org/10.1016/J.GENE.2013.03.027