- Patient presentation
- History
- Differential Diagnosis
- Examination
- Investigations
- Discussion
- Treatment
- Outcome
- Final outcome
- Evaluation - Questions & answers
- MCQ
- References
Patient presentation
- HIV positive 11 years and 10 months old male, vertically infected from his mother.
- WHO stage IV with Immunological stage 3 disease.
- Patient was treated with dual therapy for 1 month and then developed severe gastroenteritis and vomiting.
- Unable to tolerate oral fluids he was admitted to hospital
Acknowledgement
This case study was kindly provided by Dr Lee Fairlie (nee Kleynhans) MBChB DCH FCpaeds(SA) Wits Paediatric HIV Clinics, Chris Hani Baragwanath Hospital
History
In March 2006 an 11 years and 9 months old patient presented to private GP after recurrent hospital admissions for pneumonia. At this time he weighed 23 kg (<3rd percentile; 57% expected weight for age) and was classified as wasted.
Tests conducted:
- CD4=40 (absolute count)
- Normal CXR
- PPD negative
- Sputum AFB’s negative
TB excluded based on these results.
Patient was started on dual therapy:
- AZT 100mg po bd
- 3TC 5ml po bd
GP was already treating mom for HIV with HAART. She was also classified as WHO stage IV with Immunological stage 3 disease and TB was excluded.
Differential Diagnosis
- Viral or bacterial gastroenteritis made worse by underlying chronic HIV-related diarrhoea, possible causative organisms include Cryptosporidium parvum/ Giardia lamblia/ Isospora beli.
- Immune Reconstitution Inflammatory Syndrome (IRIS) – based on rapid deterioration after 1 month of starting ART.
- Opportunistic infections such as MAC (Mycobacterium Avium Complex).
- Poor food, drug and nutrient absorption because of HIV related diarrhoea.
- Lactic acidosis (although unlikely because he was not on treatment long enough and AZT does not typically cause this).
- Drug toxicity.
Examination
On admission to hospital patient was
- Moribund
- Wasted
- Dehydrated 10%
There was a strong clinical suspicion of hypokalaemia based on:
- Reduced tone, reflexes difficult to elicit.
- Distended abdomen with scanty bowel sounds suggesting a hypokalaemic ileus.
The rest of the examination was non-contributory.
Investigations
- K+ = 1mmol/L (3.3-5.0 mmol/L)
- Rest of U&E = normal
- Lactate = normal
- Mild transaminitis
Discussion
The GIT is responsible for the process of food digestion, nutrient absorption and excretion of waste. The stomach serves as the food reserve but most of the food and drug absorption take place in the small intestine which is the largest part of the GIT. The small intestine has convoluted structures that are known as villi and microvilli, which are finger like structures protruding from the lumen, increasing the surface of the mucosa for nutrient absorption (Lange, 2011). The mucosa of the GIT consists of specialized epithelial (goblet cells) cells that secretes a thick protective layer of mucous. The mucosa is responsible for nutrient absorption and transport, tissue lubrication, and protection against pathogen and foreign particles. The apical of the villi are lined with enterocytes that has microvilli on the luminal side of the cell that plays an important role in increasing the surface area for nutrient absorption (Hua, 2020; Lange, 2011).
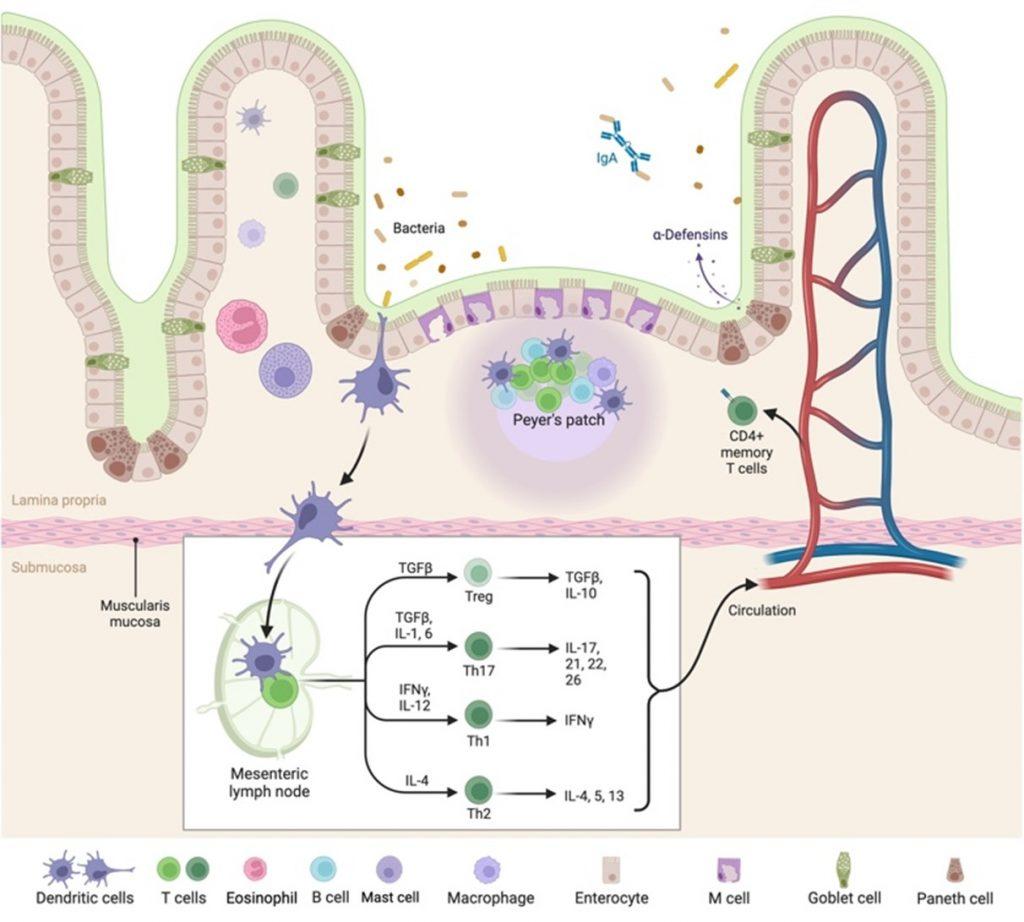
Figure 1. Anatomy of the intestinal mucosa and its immune apparatus. The small intestine as illustrated, has long thin villi that are covered by surface epithelium with an extensive brush border comprising of microvilli. This provides the large surface area to facilitate digestion and absorption of metabolites. The central part of the villus is called the lamina propria, that in which majority of the intestinal immune cells are found. As you progress down the GIT the villus become progressively shorter which directly impacts the absorption capacity. The image illustrates antigen sampling by M cells and the process of transcytosis of the antigen to the lymphoid follicle. Alternative antigen sampling is depicted as well, like direct antigen uptake by dendritic cells. Created with BioRender.com (CA Petersen 2023)
During an HIV infection the GIT mucosal barrier is the primary site for viral transmission. GIT enteropathy is characterised by increased GIT inflammation, diarrhoea, intestinal permeability and malabsorption. The most common reason for malabsorption and diarrhoea relates to opportunistic infection and antiretroviral medication (ARV). Malnutrition is observed when there is an infection within the small intestine (jejunum) also known as enteritis(Kong et al., 2018). Enteritis in turn leads to disruption in the development of intestinal villi due to local activation of the GIT immune system producing high levels of proinflammatory mediators [IL–6, IL-10, IFN–y] that directly correlates to the level of viral replication, lymphocyte infiltration is then observed, damaging the GIT epithelial layer (Brenchley & Douek, 2008), leading to villous atrophy and an increase in gut permeability to microbial organisms.
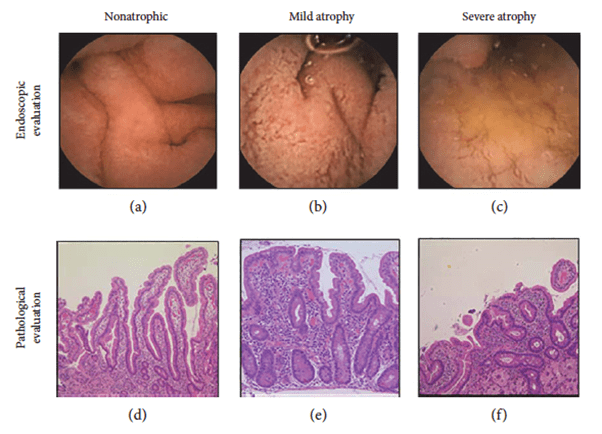
Figure 2. Representative images of villous atrophy. Villous atrophy was endoscopically diagnosed as positive when the reduction or absence of Kerckring’s folds, a mosaic mucosal pattern and scalloping, was confirmed in the duodenum (a–c). A biopsy was performed at the lower
duodenum, and villous atrophy was evaluated using the modified Marsh classification [17] (d–f). Marsh stage ≥ 3 was diagnosed as villous atrophy positive. (a) Nonatrophic villi. (b) Reduction of villi. (c) Mosaic pattern of mucosa. (d) Marsh 0. (e) Marsh 3a. (f) Marsh 3c. (Sakai et al., 2017)
It has been postulated that bacterial translocation across the damaged epithelial barrier results in local activation of the immune system, which in turn augments HIV replication due to the increase in CD4+ T Cells. It estimated that nearly 80% of the T cells population is found in the GIT, which leads to significant depletion of CD4+ cells in the first two to three weeks post HIV infection and it has been shown that patients with a CD4 T cell count less than 200 cells/ul has a twofold increase in diarrhoea. The depletion of Th17 cells from the gut-associated lymphoid tissue (GALT) due to HIV infection has been found to negatively affect the integrity of the mucosal lining and innate defence mechanisms against gut microbes. Th17 cells play a crucial role in epithelial regeneration and the production of claudins, which are essential components of tight junctions in the epithelial barrier.
The consequences are thus a reduction in absorptive surface area, disruption in enterocytes development and reduced retention of nutrient due to high volumes of diarrhoea leading to malabsorption of nutrients and drugs (Brenchley & Douek, 2008).
Treatment and management
In Hospital
- Patient was admitted to High Care where he was given intravenous potassium
- During his hospital stay he missed ARV’s for 1 day
- After patient’s potassium recovered, he was transferred to Hospice for respite care and psychosocial intervention
At Hospice
- In hospice he received nasogastric tube (NGT) feeds in the form of Peptamen, a peptide-based elemental diet for the nutritional support of GI-impaired children. This is absorbed much more easily than food and helps the gut recover, thereby increasing food and drug absorption.
- Two months on dual therapy his CD4 count increased to 117 and viral load was undetectable.
Treatment Evaluation
The ARVs he was on were noted to be an inadequate dose for the patient:
- AZT – 100mg po bd (inadequate dose, should be 200mg bd for patient’s weight)
- 3TC – 5ml po bd (should have been on 8 ml bd)
- No 3rd drug had been added
Treatment and follow-up
At this stage the question was what to do with his ARV’s? Although the therapy he was on was working as evidenced by his climbing CD4 count, suppressed viral load and general health improvement, the likelihood of resistance occurring if this is used as long term treatment is high.
The decision was made to start him on triple therapy using corrected dosages appropriate for his body weight. He continued with AZT and 3TC and efavirenz was added to the regimen because his CD4 was improving and he was virally suppressed.
After two weeks he was transferred from Hospice to a hospital close to where his mother works. He has continued with this regimen and he has been followed up regularly.
Outcome
- His progress has remained very good, his mother is still motivated and compliant and he has continued his triple therapy without interruptions.
- His weight has steadily increased to 34.5 kg (just below 3rd percentile)
- He has been clinically well and has required no intermittent admissions.
- His CD4 count has continued to increase with the last CD4 count at 160 (10.2%)
- Viral load has remained undetectable at < 25 copies
Final outcome
One year later the patient continues to do excellently on triple therapy, he is gaining weight, he has maintained viral load suppression and his CD4 count continues to increase. He is followed up regularly at the same hospital and his mother has remained compliant with his therapy and appears to be compliant with her own therapy too.
Evaluation – Questions & answers
What is the most likely diagnosis for this case?
How does the immune system function in a normal functioning GIT?
In the GIT we have a number of commensal organisms and specialized cells that produce antimicrobial agents in defence and immunity. Commensal bacteria are the first line of defence, essential to maintaining epithelial barrier integrity by producing and inducing antimicrobial factors protecting the GIT against enteric pathogens. They also interact and modulate immune responses elicited by innate immune systems located between enterocytes (inter epithelial lymphocytes), and beneath in the lamina propria (Montalban-Arques et al., 2018). Within the lamina propria there are many different innate immune cells, there are mononuclear phagocytes (macrophages and dendritic cells) that plays a role in the uptake and presentation of antigens in the intestine, mast cells and eosinophils that are normally involved in allergic reactions or parasitic worms. There are the crypts of Lieberkuhn that contains multi-potent stem cells that give rise to different types of mature epithelial cells. At the base of the crypt there are paneth cells that differentiate from stem cells to produce antimicrobial peptides following immune system stimulation, protecting the stem cells. They also play a role in maintaining normal crypt stem cell activity and are crucial in small intestine homeostasis (Mowat & Agace, 2014). The small intestine also carries out luminal antigen sampling in secondary lymphoid tissue known as Peyer’s Patches, that are characterised by overlying microfold (M cells) specialized epithelial cells for uptake and transport of particulate antigens from the lumen to dendritic cell (DC) rich environments via a process called transcytosis, where they can be presented to the adaptive immune cells (fig.1). Antigen presentation and activation of T and B cells occurs in the lymphoid follicle in close proximity to the epithelial cell layer.
How does HIV affect the absorbtion of nutrients and drugs from the GIT?
How does HIV affect the immune system in the GIT?
During an HIV infection the GIT mucosal barrier is the primary site for viral transmission. GIT enteropathy is characterised by increased GIT inflammation, diarrhoea, intestinal permeability and malabsorption. Local activation of the GIT immune system produces high levels of proinflammatory mediators [IL–6, IL-10, IFN–y] that directly correlates to the level of viral replication as this leads to an increase in gut permeability to microbial organisms. It has been postulated that bacterial translocation across the damaged epithelial barrier results in local activation of the immune system, which in turn augments HIV replication due to the increase in CD4+ T Cells. It estimated that nearly 80% of the T cells population is found in the GIT, which leads to significant depletion of CD4+ cells in the first two to three weeks post HIV infection. It has been shown that patients with a CD4 T cell count less than 200 cells/ul have been found to have a two-fold increase in diarrhoea. The depletion of Th17 cells from the gut-associated lymphoid tissue (GALT) due to HIV infection has been found to negatively affect the integrity of the mucosal lining and innate defence mechanisms against gut microbes. Th17 cells play a crucial role in epithelial regeneration and the production of claudins, which are essential components of tight junctions in the epithelial barrier.
In chronic HIV infection, immune activation encompasses various processes, including the activation of multiple B cells, elevated levels of cytokines and other proinflammatory molecules. These factors contribute to lymph node fibrosis, clonal exhaustion, and the depletion of memory T-cell populations. Consequently, antigen presentation in Peyer’s patches is significantly reduced(Afr & Clin Nutr, 2010).
These effects occur due to the failure in maintaining immune cell homeostasis within the lymphoid follicle, leading to structural and functional impairments of the tissue.
Moreover, the depletion of CD4 T helper cells in the Lamina propria hinders the recruitment of new T and B lymphocytes into the lymphoid tissue. Consequently, the physical size of Peyer’s patches is reduced due to the absence of immune cell proliferation in the lymphoid follicle.
What are the benefits of triple therapy over dual therapy?
The initiation of combined antiretroviral therapy (cART) leads to rapid suppression of the virus and a notable decrease in immune system activity, resulting in the recovery of CD4+ cells.
According to a study conducted by Van Welzan et al., dual therapy is comparable to triple therapy in terms of virological outcomes and restoration of CD4+ cell counts. However, it’s important to note that these findings are based on 48-week trial and may not accurately reflect long-term treatment effectiveness or failure when comparing the two therapy strategies (van Welzen et al., 2021).
Dual therapy offers several advantages, including the potential to minimize long-term toxicity, improve tolerability, enhance adherence, and reduce costs associated with treatment.
However, limited evidence suggests that dual therapy may not effectively address the persistent immune activation caused by viral replication in reservoir sites such as the gastrointestinal tract (GIT). In these sites, high levels of multi-drug resistant proteins continue to exist, potentially leading to chronic low-grade inflammation of the mucosal lining. This inflammatory state can hinder complete restoration of CD4+ cell counts and may also impact the absorption of nutrients and medications (Baril et al., 2016).
The benefit of using triple therapy is that there is more literature advocating that HAART reduces opportunistic infections and cancers and decreases death by more than 50 % with minimal treatment failure.
Multiple Choice Questions
Earn 1 HPCSA or 0.25 SACNASP CPD Points – Online Quiz
References
- Afr, S., & Clin Nutr, J. (n.d.). The gastrointestinal tract and HIV pathogenesis.
- Baril, J. G., Angel, J. B., John Gill, M., Gathe, J., Cahn, P., Van Wyk, J., & Walmsley, S. (2016). Dual Therapy Treatment Strategies for the Management of Patients Infected with HIV: A Systematic Review of Current Evidence in ARV-Naive or ARV-Experienced, Virologically Suppressed Patients. PLoS ONE, 11(2). https://doi.org/10.1371/JOURNAL.PONE.0148231
- Brenchley, J. M., & Douek, D. C. (2008). HIV infection and the gastrointestinal immune system. Mucosal Immunology, 1(1), 23–30. https://doi.org/10.1038/MI.2007.1
- Hua, S. (2020). Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract – Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Frontiers in Pharmacology, 11, 496058. https://doi.org/10.3389/FPHAR.2020.00524/BIBTEX
- Kong, S., Zhang, Y. H., & Zhang, W. (2018). Regulation of intestinal epithelial cells properties and functions by amino acids. BioMed Research International, 2018. https://doi.org/10.1155/2018/2819154
- Lange, K. (2011). Fundamental role of microvilli in the main functions of differentiated cells: Outline of an universal regulating and signaling system at the cell periphery. Journal of Cellular Physiology, 226(4), 896–927. https://doi.org/10.1002/JCP.22302
- Montalban-Arques, A., Chaparro, M., Gisbert, J. P., & Bernardo, D. (2018). The Innate Immune System in the Gastrointestinal Tract: Role of Intraepithelial Lymphocytes and Lamina Propria Innate Lymphoid Cells in Intestinal Inflammation. Inflammatory Bowel Diseases, 24(9), 1649–1659. https://doi.org/10.1093/IBD/IZY177
- Mowat, A. M., & Agace, W. W. (2014). Regional specialization within the intestinal immune system. In Nature Reviews Immunology (Vol. 14, Issue 10, pp. 667–685). Nature Publishing Group. https://doi.org/10.1038/nri3738
- Sakai, E., Higurashi, T., Ohkubo, H., Hosono, K., Ueda, A., Matsuhashi, N., & Nakajima, A. (2017). Investigation of Small Bowel Abnormalities in HIV-Infected Patients Using Capsule Endoscopy. Gastroenterology Research and Practice, 2017. https://doi.org/10.1155/2017/1932647
- van Welzen, B. J., Oomen, P. G. A., & Hoepelman, A. I. M. (2021). Dual Antiretroviral Therapy—All Quiet Beneath the Surface? Frontiers in Immunology, 12, 637910. https://doi.org/10.3389/FIMMU.2021.637910/BIBTEX










