Introduction
- In a clinical setting, the term transplantation generally refers to the transfer or replacement of cells, tissues, or organs from one individual to another.
- The source of the transplant can either be a person’s own body, another human, or another species.
- In the human, with the exception of transplantation of one’s own tissues, where the recognition of ‘‘self’’ components evokes no immune response, the transplantation of all other tissues, i.e., from another human or another species, triggers immune responses that are similar to those mounted against foreign substances and that can be complex and at times evoke severe complications.
- Transplants are broadly classified as solid organ or hematopoietic stem cell transplants and within these classifications are categorized according to the relationship of the donor and the recipient.
Transplant Immunity
- A variety of terms are used to define antigens and immune responses stimulated in a transplant setting according to the relationship of the donor and the recipient.
- The tissues transplanted from the same individual (i.e., autografts) are termed autologous transplants since they contain autoantigens or ‘‘self’’ antigens and under normal circumstances evoke no immune responses.
- An auto-graft is a transplant of self-tissue or cells, e.g., skin or hematopoietic stem cells (HSC).
- Autologous skin transplants are often performed on burn patients, and autologous HSC transplantation is commonly used as a means of rescuing cancer patients subsequent to myeloablative chemotherapy.
- The term isograft is sometimes used when referring to a syngeneic transplant between two different genetically identical individuals.
- In humans, only a transplant involving identical twins would be a true syngeneic isograft.
- In an animal model, a syngeneic transplant would involve two animals derived from the same inbred strain.
- The term allogeneic graft refers to a transplant that involves the transfer of an allograft containing alloantigens, which are genetically controlled characteristics that differentiate one member of a given species from another.
- The vast majority of transplants carried out in humans are allogeneic.
- However, due to the serious shortage of donor organs, researchers are actively investigating ways to successfully perform xenotranplantation, which involves the transfer of organs or tissues, i.e., a xenograft, containing xenoantigens between members of two different species.
The Immune Responses Involved in Transplantation
- The immune responses involved in transplantation are governed by the laws that are based on the genetics of the donor and recipient
- Figure 1 shows a schematic representation of the genetic controls affecting transplantation in a mouse model system in which a variety of transplants are conducted either between members of two genetically identical (syngeneic) mouse strains, or from one member of one inbred species (allogeneic) to another or from a foreign species (xenogeneic) to one of the inbred mouse strains.
- In this model, there are the following participants: (1) a genetically inbred white mouse strain (Strain A) containing homozygous MHCa; (2) a genetically inbred black mouse strain (Strain B) containing homozygous MHCb; (3) an F1 hybrid offspring (F1 Strain A/B) resulting from the crossmating of the two parental types, the offspring of which contain genetic characteristics from both parents as a heterozygote MHCa,b; and (4) a guinea pig representing a member of a foreign species.
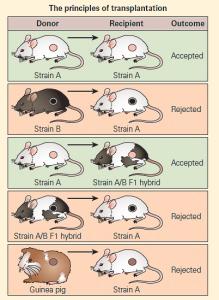
Figure 1. Schematic representation of the genetic controls affecting transplantation in a mouse model system. [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
- A skin graft from a guinea pig to either parental strain will be rejected as a foreign graft (xenograft).
- A skin graft from a member of Strain A to another member of Strain A (or from Strain B to Strain B) will be accepted.
- A skin graft from a member of Strain B to a member of Strain A, or vice versa, will be rejected.
- A skin graft from Strain A transferred to the F1 hybrid will be accepted because the hybrid recognizes, at least in part, one half of the genetic makeup of the transplant as self antigen and will not evoke rejection.
- A skin graft from the F1 hybrid to either parental strain, on the other hand, will be rejected since the recipient will recognize only one half of the genetic identity of the donated tissue as self and one half as foreign.
- The term ‘‘alloimmunity’’ is used to refer to the immune response stimulated by an alloantigen.
- An alloimmune response can involve the two modes of antigen processing described in (Figure 2), i.e., either the direct recognition of nonself human leukocyte antigen (HLA nonself) (the ‘‘exogenous’’ pathway; ie, MHC-II) or the indirect recognition of an alloantigen-derived peptide presented by self-HLA (the ‘‘endogenous’’ pathway; ie, MHC-I).
- Sensitization to alloantigens can occur in a variety of clinical settings, including transplantation, pregnancy, and blood transfusions. Once sensitized, many individuals develop potent antibodies and cell-mediated immune responses specific for HLA antigens.
- In a transplant recipient who has never been previously sensitized to histocompatibility antigens, the first step of the immune response requires the recognition of the donor’s (nonself) HLA antigens by the T cell receptor on the recipient’s alloreactive T lymphocytes.
- This interaction triggers a cascade of specific and nonspecific immune events that ultimately lead to rejection of the transplanted organ.
- Transplant rejection is caused primarily by a cell-mediated immune response to HLA antigens expressed on donor antigen-presenting cells (APCs) transferred along with the transplanted organ.
- The process of graft rejection can be divided into two phases: (1) a sensitization phase in which antigen reactive lymphocytes in the recipient’s lymph node proliferate in response to the donor’s alloantigens and (2) an effector phase in which the recipient’s sensitized effector cells mediate immune destruction of the graft.
- For initial sensitization to take place, CD4+ and CD8+ T cells must proliferate as a result of the recognition of nonself HLA antigens.
- The response to major histocompatibility antigens involves recognition of both the HLA molecule and an associated peptide ligand in the cleft of the HLA molecule.
- Activation of host CD4+ T helper cells requires interaction with an APC expressing an appropriate peptide-HLA molecule complex along with a requisite co-stimulatory signal (Figure 2).
- Recognition of donor HLA antigens on the cells of the graft induces vigorous T cell proliferation in the recipient.
- Both dendritic cells and vascular endothelial cells from the donor organ induce vigorous proliferation of recipient T cells in this reaction.
- The major proliferating cell is the CD4+ T cell, which recognizes HLA class II antigens directly or HLA peptides presented by host APCs.
- This amplified population of activated T helper cells plays a central role in the induction of the various effector mechanisms involved in graft rejection.
- There are two potential mechanisms by which a solid organ is rejected as a result of alloreactivity, i.e., immunogically mediated response to antigens that are distinct between members of the same species (Figure 2).
- The first mechanism of graft rejection occurs by direct recognition of the graft. This takes place when MHC molecules shed from the graft on recipient antigen-presenting cells are recognized by recipient T cells in regional lymphoid tissue (Figure 2A).
- This occurs without respect to any donor peptide that may be in the cleft of the donor MHC but requires both the presence of APCs in the graft as well as adequate lymphatic drainage to regional lymph nodes.
- Presentation of the foreign MHC and generation of an appropriate co-stimulatory signal results in activation of the recipient T cells with rejection of the graft (Figure 2A).
- A second mechanism of graft rejection occurs by indirect recognition of the graft. Trauma from the transplant itself can occur during the procedure, with release of foreign proteins from the graft that are taken up and processed by recipient APCs resulting from the pro-inflammatory environment. Following peptide presentation and appropriate co-stimulatory signals, the recipient T cells are activated with rejection of the graft (Figure 2B). Approximately 1 to 7 percent of naıve T cells are capable of responding to alloantigens by either mechanism.
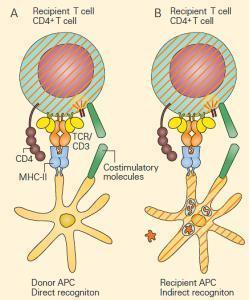
Figure 2. Alloreactivity: direct and indirect responses to alloan-tigen. Panel A: Direct alloresponses rely on the T cell receptor on recipient T cells recognizing the MHC of donor antigen-presenting cells (APCs) as foreign. This occurs without respect to the peptide in the cleft. Following migration of donor APCs to regional lymph-oid tissue of the recipient, the foreign MHC is recognized by the TCR of the recipient’s T cells with subsequent T cell activation and graft rejection. Panel B: In indirect alloresponses following trauma, foreign antigens are released from the graft and are processed by recipient APCs and the resultant peptides are presented to recipient T cells with subsequent T cell activation and graft rejection. In both cases, co-stimulation is required. [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].The most common of these are cell-mediated reactions involving delayed-type hypersensitivity and cytotoxic T lymphocyte (CTL) mediated cytotoxicity.
- In addition, complement-dependent antibody lysis or natural killer (NK) destruction by antibody-dependent cell-mediated cytotoxicity (ADCC) can occur.
- The hallmark of cell-mediated graft rejection is an influx of T cells and macrophages into the graft.
- Histologically, the infiltration often resembles that seen during a delayed-type hypersensitivity response in which cytokines produced by Th1 cells promote macrophage infiltration.
- Recognition of donor HLA class I antigens on the graft by recipient CD8+ cells can lead to CTL-mediated killing. In some cases, graft rejection can also be mediated by CD4+ T cells that function as HLA class II restricted cytotoxic cells.
- In each of the effector mechanisms, cytokines secreted by T helper cells play a central role (Figure 3). For example, IL-2, IFN-g, and TNF-a have each been shown to be important mediators of graft rejection.
- IL-2 promotes T cell proliferation and generally is necessary for the generation of effector CTLs.
- IFN-g is central to the development of the delayed-type hypersensitivity Th1 response and in promoting the influx of macrophages into the graft with their subsequent activation into more destructive cells.
- TNF-a has been shown to have direct cytotoxic activity on cells of a graft exerting its effects by apoptosis. In addition, a number of cytokines promote graft rejection by inducing expression of HLA class I or class II molecules on engrafted cells.
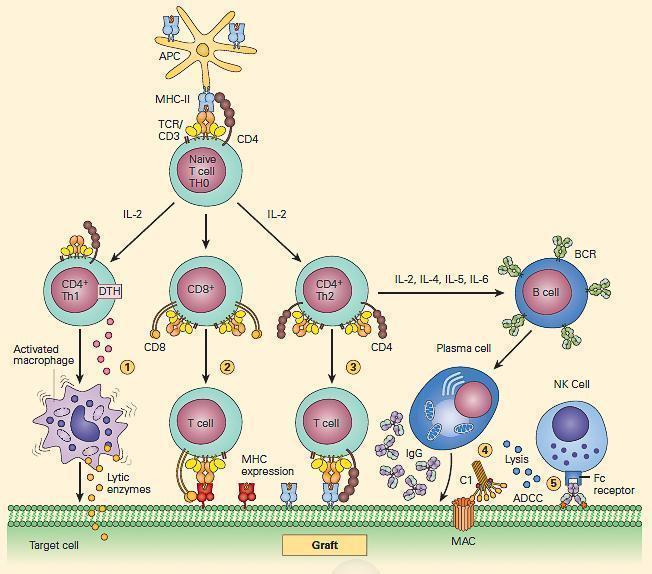
Figure 3. Schematic representation of the cellular events involved in antigen recognition and immune reactivity associated with graft recognition. [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
The Determining Role of Inflammation in Shaping Lineage Commitment of CD4+ Naive T Cells in Engrafted Organs
- Newly engrafted organs are often subject to intense inflammation resulting from several causes, including donor disease, inappropriate organ procurement, preservation in nonphysiological fluids, surgical trauma, or reperfusion injury.
- The inflammatory response, in turn, leads to release of proinflammatory cytokines such as IL-6, TNF-a, and IL-1b.
- The lineage commitment of naive recipient CD4+ T cells in the graft is determined by the cytokine environment in which these cells recognize donor antigens.
- Thus, depending on the nature of the cytokine milieu present when antigen activation occurs, naive recipient CD4+ helper T cells can differentiate along diverse developmental cellular pathways with either cytopathic and/or immunoregulatory functions (Figure 3).
- When CD4+ T cells are activated in the presence of IL-12, usually produced by activated, mature DCs, they differentiate into IFN-g-producing Th1 cells.
- In contrast, when CD4+ naive T cells are activated in the presence of IL-4, they differentiate into Th2 cells, which produce IL-4, IL-5, and IL-13.
- In the absence of proinflammatory cytokines, TGF-b induces differentiation of CD4+ T cells into expression of FOXP3+
- In contrast, expression of TGF-b with IL-6 or IL-21 prevents development of the transplant-protective effects of Tregs; instead, the antigen-reactive CD4+ T cells become IL-17-producing Th17 cells, which are highly cytopathic (Figure 4).
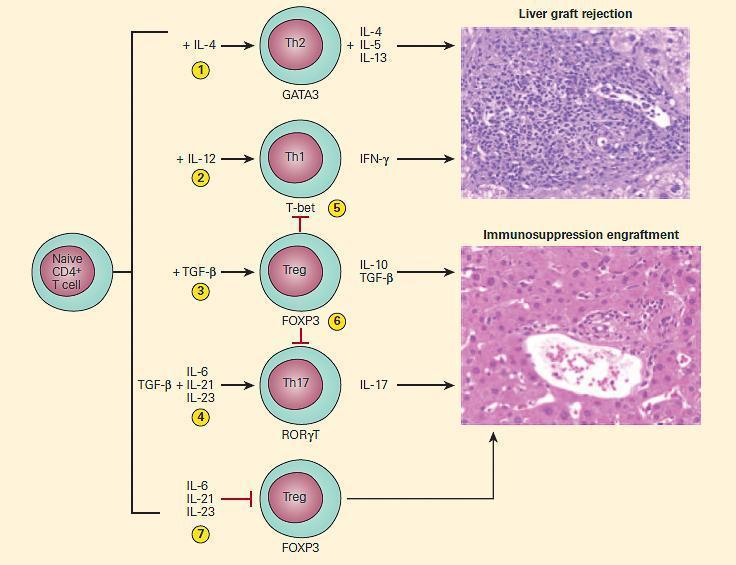
Figure 4. Schematic representation of the effects of cytokine environment on lineage commitment of naive CD4+ T cells in determining outcome of liver transplantation. Upon activation by cognate antigens and costimulatory signals, recipient naive CD4+ T cells acquire graft-destructive or graft-protective phenotypes, depending on the local cytokine microenvironment in which T cell activation occurs. In the presence of IL-4, CD4+ T cells become Th2 (1) and in the presence of IL-12 they become Th1 cells (2). The presence of TGF-b, in the absence of proinflammatory cytokines, induces differentiation of recipient CD4+ T cells into tissue-protective FOXP3+ Treg cells (3); in contrast, TGF-b in the presence of IL-6, IL-21, or IL-23 induces the differentiation of CD4+ naive T cells into the Th17 phenotype (4). The Treg cells can inhibit the induction of tissue-destructive Th1 (5) or Th17 (6) cells. The presence of IL-6, IL-21, or IL-23 blocks the generation of Tregs (7) that can further enhance graft rejection. (Adapted and reproduced with permission from Sa´nchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology. 2011;140:51–64.) [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
Relative Balance Between Rejection-Prone Effector T Cells and Rejection-Blocking, Immunosuppressive Tregs
- Until recently, it was believed that recipient naive T cells activated by donor antigens became terminally differentiated Th1 or Th2 cells that had opposing effects, i.e., Th1-dependent responses were thought to generate cytopathic graft rejection and Th2-dependent responses cytoprotective effects.
- However, this previously held Th1/Th2 paradigm was incorrect since it was subsequently shown that both Th1 and Th2 can each mediate graft rejection (Figure 4).
- Treg cells are now accepted as the key arbiters of immunologic tolerance that determine whether a graft will undergo cytopathic graft rejection or be maintained in a tolerant cytoprotective state.
- The currently accepted paradigm is that the outcome of rejection or graft acceptance is ultimately determined by the relative balance between cytopathic Th1 and Th17 CD4+ T cells versus rejection-blocking, cytoprotective regulatory T cells.
- This balance, in turn, depends on the qualitative levels of cytokines in the inflammatory microenvironment in which T cell activation takes place.
Clinical Manifestations and Treatment
- Solid Organ Transplantation Immunosuppressive medications have revolutionized the field of transplantation.
- Generally, these drugs either globally suppress T cell reactivity or lead to the death of reactive T cells. These agents are required for any form of transplantation across MHC barriers.
- In the case of solid organ transplantation, organs are fully (living donor) or partially (living donor or cadaveric donor) HLA matched for the recipient.
- For kidney transplants, the overall rate of graft failure in the first year is 10 percent, and the overall rate of graft failure at five years is 35 percent (see Chapter 22 in Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012]).
- The likelihood of graft rejection is increased with increasing MHC disparity.
- In addition to MHC matching, there are two additional compatibility tests that are performed for renal-transplant recipients. Kidneys are also matched for ABO blood group antigens.
- When kidneys are transplanted into ABO-incompatible recipients, only 50 percent of the kidneys function at one year post-transplant.
- The last level of matching that is performed is called cross-matching. This assay determines whether there is preexisting antibody in the recipient to donor histocompatibility antigens. The determination of antibody to ABO blood groups or donor MHC antigens is important for preventing graft rejection. This is generally a problem with patients who have received more than one graft, but also can be seen in patients receiving their first graft.










