Introduction
- The maternal-foetal interface of the placenta is a complex microenvironment containing soluble and cellular regulatory factors necessary for the establishment and maintenance of a normal pregnancy.
- Regulation of these factors is precisely localized, and changes as the pregnancy progresses.
- The placenta is both an immune-privileged site and the conduit through which the foetus receives all nutrition and oxygen necessary for development, as well as soluble maternal immune factors.
- The placenta is of foetal origin and invades the uterus diverting maternal blood flow.
- Within the placenta, maternal blood bathes the foetal villi allowing for exchange of gases, nutrients, wastes and soluble immune factors between the two vasculatures.
- The foetus and placenta express paternal antigens. Although recognized by the maternal immune system, changes in both the systemic and local (uterine) immune responses abrogate normal cytotoxic adaptive reactions while enhancing regulatory, tolerogenic responses allowing the pregnancy to progress.
- Any breakdown in immune tolerance can result in pregnancy-specific disease.
- Human pregnancy requires a unique immunological setting in which the foetal allograft is tolerated and maintained within the maternal immune system.
- Imbalance in this dynamic and tightly controlled interaction has been implicated in adverse pregnancy outcomes (recurrent pregnancy loss, pregnancy-induced hypertension, intra-uterine growth restriction, premature labour).
- The placenta (which is of foetal origin) is the interface at which two immunologically distinct individuals connect, and the site at which pathology is evident.
- Transfer of maternal IgG across the placenta provides some protection for the neonate before the development of an active infant immune system.
- This is an active process mediated by the MHC class I-like Fc receptor (FcRn) expressed on syncytiotrophoblast cells, and is dependent on total maternal IgG and specific antibody subclasses.
- Any immune imbalance at the maternal-foetal interface has implications for the developing foetus
Immune cells in the placenta
- During pregnancy there is a shift from a Th1 to Th2 profile with evidence of increased activation of innate cells in the systemic circulation.
- Regulatory CD4+CD25+Foxp3+ T cells (Tregs) expand during the second and third trimesters of pregnancy in the peripheral blood and in the decidua, believed to be induced by paternal antigens and contributing to the local control of foetus-specific maternal immune responses.
- Within the uterus, there is a redistribution of leucocytes, the functions of which are locally controlled by the decidua.
- Uterine natural killer (uNK) cells (CD56brightCD16dim) are the dominant cell type (up to 70%) with smaller populations of macrophages (20%) and lymphocytes.
- These cells are thought to influence trophoblast invasion (ingrowth of the placenta into the endometrium of the uterus and vascular modelling of the spiral arteries.
- The majority of the macrophages express CD209 (DC-SIGN) and are the main antigen-presenting cells in the uterus, also demonstrating an inhibitory phenotype.
- Fifty percent of cells in the decidua are of maternal origin
- The maternal-foetal interface in the decidua is rich in activated CD8, activated Tregs and classically activated macrophages
Interplay of cells at the maternal-foetal interface
- The maternal–foetal interface is composed of foetal trophoblasts intermingled with maternal leukocytes, stromal, and endothelial cells that comprise the decidua
- During implantation, trophoblasts, derived from the trophoectoderm surrounding the blastocyst, differentiate into the syncytiotrophoblasts that infiltrates the endometrium, and the cytotrophoblasts at the embryo side.
- The layer of syncytiotrophoblasts in contact with the decidua represents the extravillous trophoblasts (EVTs)
- EVTs orchestrate bi-directional cross-talk between the mother and the foetus by providing structural and biochemical barriers,
- They serve as an endocrine organ that support and regulate placental and foetal development, and modulate maternal innate and adaptive immune responses
- EVTs express and secrete HLA-G and release IL-10, which instruct decidua APC to become tolerogenic DC secfeting IL-10 and promoting the induction of a variety of Tregs (both Tr1 cells, CD4+CD25+FOXP3+ Treg and CD4+HLA-G+ Tregs) – shown in Figure 1
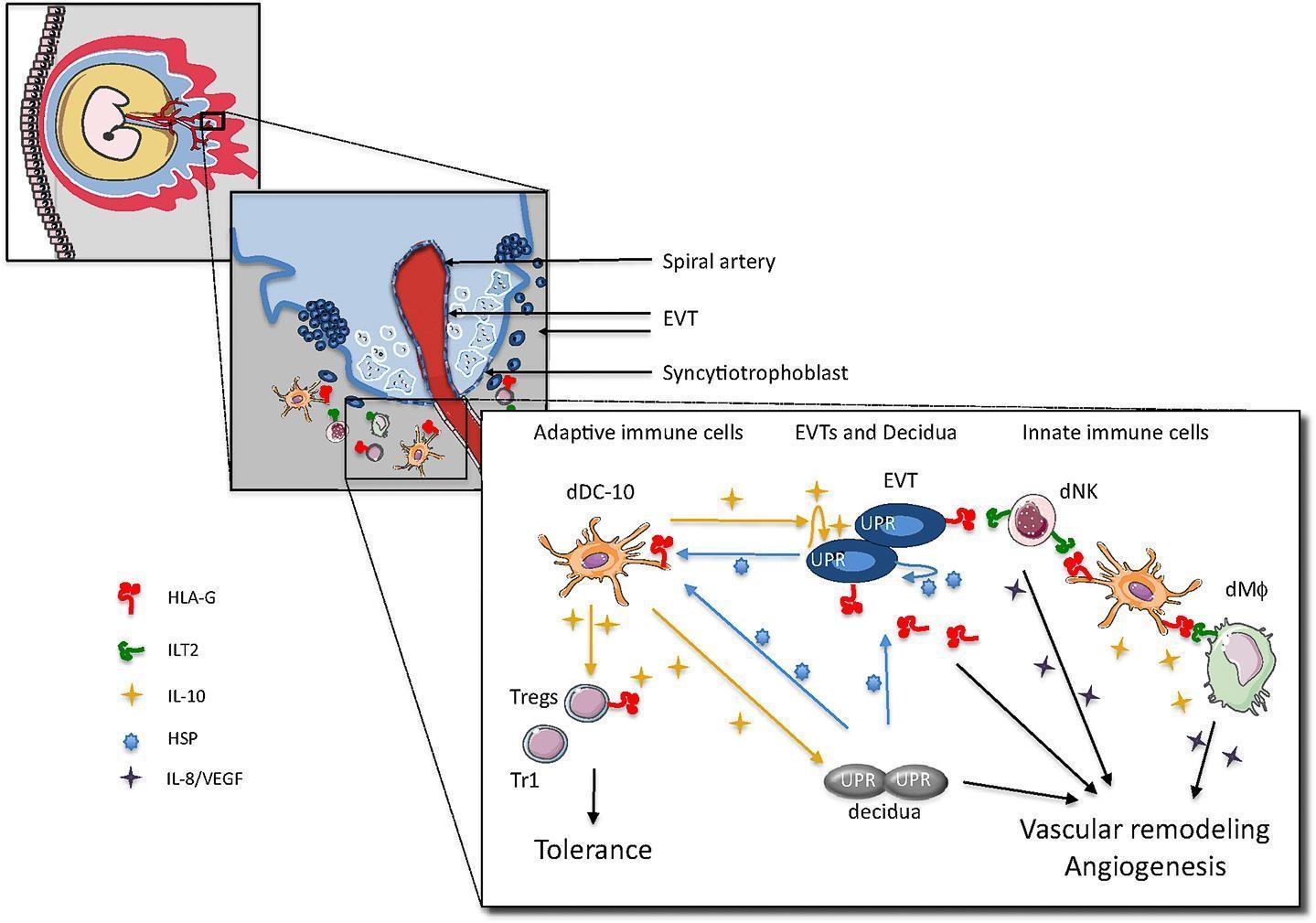
Figure 1: Proposed model for cross-talk among embryo trophoblasts, decidual leukocytes and stromal cells at the maternal-foetal interface in the human first trimester of pregnancy. [Gregori S, et al. HLA-G orchestrates the early interaction of human trophoblasts with the maternal niche. Frontiers in Immunology 2015; 6(128)]
- Induced Treg inhibit effector T cells and via IL-10 secretion, promote HLA-G expression on EVTs
- EVTs via HLA-G directlt promote decidual NK cell activation and the release of angiogenic factors
- dDC-10 is HLA-G+ and can interact with either dNK cells or decidual macrophages via ILT2 and promotes their activation and pro-angiogenic effects
- One fundamental question in reproductive immunology, one that will impact on local immune tolerance and successful pregnancy outcome, is whether uterine NK cells and Tregs change their “pregnancy-friendly” and immune tolerant phenotype to an adverse phenotype in the setting of excessive inflammation, infection and stress.
- Mild, beneficial inflammation-like responses are normally elicited during implantation, possibly induced by semen (seminal fluid).
- Pivotal roles of inflammatory cytokines such as GM-CSF, LIF, IL-1, IL-6, and IL-11 have been implicated in successful implantation and decidualization
- However, in cases of excessive inflammation during different maternal disease states, such as with HIV infection, TB or malaria, this is one question that remains to be addressed.
Quiz
Related Talk
Clare Cutland, Wits Health Consortium – Maternal Vaccination












