Reactivity vs. Tolerance
- It is incompletely understood how mucosal immunity differentiates between pathogens and commensals or harmless environmental antigens
- Tolerance induction is mostly at play at these surfaces, as we know that APC present antigens to lymphocytes, which activate and proliferate under the influence of micro-environmental cytokines
- This involves activated T-cells interacting with cognate B-cells
- Activated lymphocytes enter the circulation to seed effector mucosal site/s where final differentiation into effector or memory cells occurs
- One mechanism of mucosal tolerance appears to be elimination of pathogens in the absence of an epithelium-damaging inflammatory response
- However, there are also regulatory cells operational in the mucosa, dampening or suppressing reactive antigen-specific cells.
Mucosal Immune Regulation
- Several levels of mucosal immune regulation exist, via a complex interplay between innate and adaptive mechanisms
- Nature of the antigen: pathogen-associated molecular patterns (PAMPs) ligate pattern-recognition receptors on innate APC, upregulating MHC and co-stimulatory molecule expression
- Replicating rather than inactive microbes are more likely to induce productive immune responses
- Soluble antigens are handled differently by tolerance pathways, which may explain the failure of clinical oral/nasal immunisation using soluble antigens
- APC: immature DC or non-professional APC (e.g. epithelia) lack co-stimulatory molecules, which may lead to T-cell anergy or apoptosis
- Mucosal cytokines: mucosal ‘accessory’ cells (DC, mast cells, eosinophils, macrophages, NK cells, γδT-cells) release preformed mediators and cytokines on antigen stimulation, priming the micro-envoironment for a particular adaptive response shape
- Such mediators can include suppressor cytokines such as IL-10 and TGFβ (e.g. Peyer’s patch DC make IL-10 but little IL-12, leading to Th2-type responses)
- DC antigen presentation and micro-environemtnal cytokines released by rapid-response cells profoundly impact the immune response shape (Th1-type cell-mediated immunity, Th2-type antibody-mediated immunity, or Treg-mediated tolerance) and outcomes
- IL-12 and IFNγ from activated macrophages can break tolerance
- Nature of the antigen: pathogen-associated molecular patterns (PAMPs) ligate pattern-recognition receptors on innate APC, upregulating MHC and co-stimulatory molecule expression
DC in Mucosal Tolerance
- DC antigen presentation regulates tolerance through: deletion of autoreactive T-cells, induction of anergy by immature DC (expressing low levels of co-stimulatory molecules), and induction and expansion of Treg
- Humans have two major DC populations: CD11chi (myeloid DC or ‘mDC’) and CD11clo (lymphoid/plasmacytoid DC or ‘pDC’)
- Activated DC influence the shape of the Th response, with Treg induced by IL-10-producing subsets
- Two pulmonary subsets of pDC induce Tr1 in vitro and in vivo
- Steady-state intestinal DC may be conditioned by epithelial factors to promote Foxp3+ Treg, which promote IgA-secretory B-cell populations in the mesenteric lymph nodes, also contributing to immunoglobulin-mediated immune exclusion (Figure 1A)
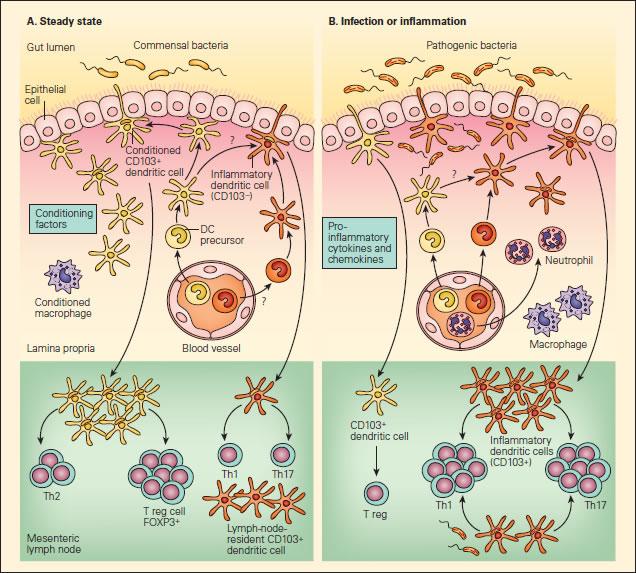
Figure 1. Panel A: Schematic representation of the interactions of DCs with T effector and Treg cell responses to commensal bacteria in the steady state. Panel B: The anti-inflammatory or proinflammatory responses in the steady state or during infection or inflammation. In the steady state, this conditioning may also occur following the sampling of commensal bacteria or alternatively to self antigens or to proteins found in food products, resulting in responses that “silence” the immune response through mechanisms of tolerance that include Treg cells or the effects of IgA-associated immune exclusion. A small number of another subset of DCs (shown in orange) may also be recruited to mesenteric lymph nodes in the steady state that may have escaped conditioning and drive T helper populations toward Th1 or Th17 profiles. Since these cells exist in small numbers, they will not give rise to disease in the steady state, but could act as sentinels when pathogenic bacteria are encountered or produced in excess, as in the case where aberrant responses to soluble proteins give rise to disease symptoms, as in the case of food allergy [Reproduced with permission from Bellanti JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
- during epithelial invasion by replicating pathogens, on the other hand, PRR activation enhances pro-inflammatory cytokine production and phagocyte recruitment (incoming naïve DC are not conditioned, and encountering pathogenic antigens thus leads to pro-inflammatory Th1 and Th17 responses) (Figure 1B)
Regulatory Lymphocytes at the Mucosa
- It is likely that tolerogenic DC within the mucosa induce Tregs
- Tregs are cells that actively control, suppress, or inhibit the function of other cells and play major roles in the pathogenesis of allergic, autoimmune, and infectious diseases and cancer (Figure 2 and Chapter 20 of Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
- CD4+ Tregs are abundant in mucosal lymphoid tissues, where they downregulate Th1 and Th2 responses.
- These cells are characterized by CD25 expression, CTLA-4 binding, and by the production of regulatory cytokines (IL-10 and TGF-β).
- Expression of CD25 on T cells is associated with natural regulatory function; CD25 is the α-chain of the IL-2 receptor and is also a marker of T cell activation.
- FOXP3 is the key regulatory gene in the development of CD25+ Tregs, which can be induced in the periphery, and their conversion into Tregs is dependent on TGF-β.
- Tr1 cells are a subtype of Tregs found in both humans and mice that are characterized by their low proliferative capacity, their production of high levels of IL-10, their production of low levels of IFN-γ, and their failure to produce IL-4.
- Another subset of Tregs, termed Th3, which produces high levels of TGF-β, plays an important role in the induction of oral tolerance.
- Tr1 cells seem to exert their suppressive activity due to their ability to produce high levels of IL-10 and TGF-β, whereas Th3 cells may act solely through the production of TGF-β, which has been claimed also to induce IgA class switching in vitro.
- The generation of active suppression or clonal anergy and/or deletion is due to the induction of TGF-β-producing Th3 cells that downregulate host responses.
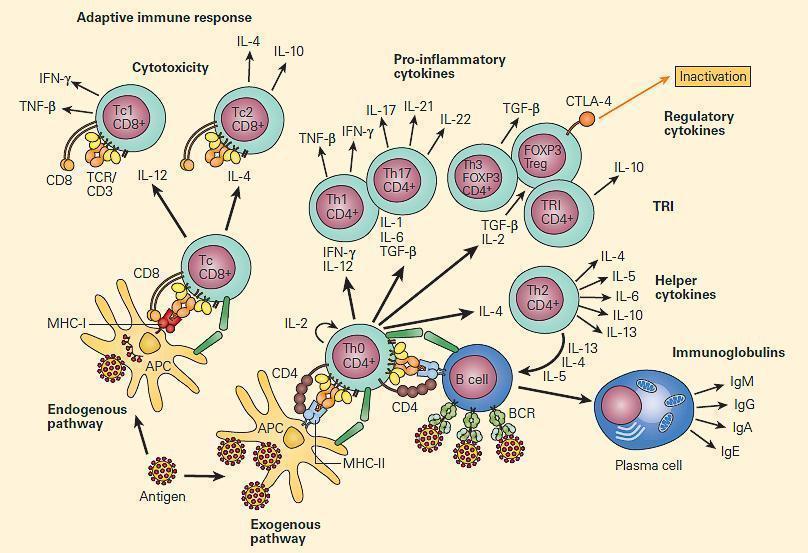
Figure 2. Immune regulation in mucosal surfaces is a complex interplay between innate and adaptive immune mechanisms. Antigen presentation by DCs and microenvironmental cytokines released by quick response cells like intraepithelial lymphocytes (IEL), mast cells (MCs), and macrophages, have a profound impact in the outcome of the immune response, either inducing cell-mediated immunity (CMI) with Th1 cells, antibody-mediated immunity through Th2 cells, or immune regulation and tolerance with T regulatory cells (Tregs). Activated macrophages are able to break tolerance by releasing IL-12 and IFN-γ. [Reproduced with permission from Bellanti JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012]
Mucosal Immunopathology
- From the above description, it would be clear that mucosal tolerance is a major immunoregulatory mechanisms maintaining host homeostasis
- Although the aetiology of most systemic and mucosal immunopathology remains unknown, there are multifactorial triggers, including genetic predisposition and the environment (e.g. microbes, pollutants)
- The current therapeutic focus is on alleviating symptoms rather than addressing the underlying cause
- Deeper knowledge of mucosal immune physiology and aetiology of immunopathology will facilitate rational manipulation of mucosal immunity for prevention and cure
- Understanding different states of pathology within mucosal tissue is important to devise therapeutic interventions.
- The following are some of the differing disease states that will result in a disruption of immune homeostasis at mucosal surfaces leading to pathology:
Inflammatory Bowel Disease
- There are two major human inflammatory bowel diseases (IBDs): a) regional ileitis (Crohn’s disease) and b) ulcerative colitis (UC)
- They are chronic, relapsing, and tissue-destructive, characterised by abdominal pain, diarrhea, bloody stool, weight loss, and intestinal inflammation
- Aetiologies are unknown, but evidence suggests a breakdown in mucosal immune tolerance to commensals most likely due to an initial insult followed by inappropriate sustained responses to commensals (see section 6: Regulation of Immunity & the microbiome).
- Altered Treg mechanisms and regulatory cytokine imbalances are seen in experimental models. These include excessive Th1 IFNγ production in response to autoantigens and commensals, Th2-type responses with IL-4 and IL-13 promoting B-cell IgE production and mast cell sensitisation, and altered mucosal Th1:Th17 balances with roles for IL-23 in intestinal homeostasis.
- A good clinical example of where there is break-down of the gut intestinal immune tract is with HIV infection
- This is associated with enhanced gut-associated lymphoid tissue Th17 and polyfunctional HIV-specific T-cell responsess (see Case Study – A case of fever and general malaise)
- Th3 can down-regulate immune responses: Treg-derived IL-10 and TGFβ may prevent colitis
- Genetic risk factors for Crohn’s diease include variations in NOD2/CARD15: studies of defective expression suggest inadequate responses by epithelia and macrophages could be the primary pathophysiological event, with T-cell activation as a secondary effect, inducing chronic inflammation
Asthma
- Allergic diseases are due to exaggerated Th2-type responses (typically involving IgE) to harmless antigens in genetically susceptible individuals
- Th1 can counter-regulate Th2, or Treg can suppress both Th1 and Th2 responses, including antigen-induced airway eosinophilia
- Childhood tolerance to cow’s milk proteins correlates with the presence of Treg, and Tr1 also prevent induction of allergic disease
- The Th1-like subset of Treg are Foxp3+T-bet+, and produce both IFNγ and IL-10, but Th1-like, Th2-like, and Tr1 Treg all suppress Th1- and Th2-type effectors via suppressive cytokines
- Allergic disease may emerge due to a deficit in function rather than number of CD4+CD25+ Treg, although impaired Treg expansion may also lead to allergy and asthma
- Immunotherapy to enhance development of allergen-specific Treg may provide safe, specific, and durable control of allergy and asthma
Food Allergy
- Food allergy is synonymous with food hypersensitivity or intolerance and describes abnormal IgE-mediated immune responses to ingested food
- It occurs in up to 8% of children under the age of three, and in ~2% of adults
- Mucosal IgE overproduction sensitises mast cells and basophils, facilitating degranulation on cross-linking by food antigens
- Infant susceptibility to food-allergic reactions may result from immaturity of the gastrointestinal barrier and immunity
- Studies suggest that breast-feeding promotes oral tolerance, preventing food allergy and atopic dermatitis in young children
IgA Deficiency
-
- This is the most common primary human immunodeficiency (1 in 300-500 people are affected)
- Although more IgA is normally synthesised daily than all other immunoglobulin isotypes combined, most affected individuals are asymptomatic (apparently due to a compensatory increase in IgM production
- Some affected individuals (possibly a subset also deficient in IgG2 and a variety of T-cell functions) do, however, have significant allergic and autoimmune diseases
- Common infections in symptomatic patients include recurrent ear infections, sinusitis, bronchitis, and pneumonia, and patients may be more susceptible to allergic disease such as asthma and food allergy
- The spectrum of autoimmunity includes rheumatoid arthritis (RA), SLE, and ITP
- Patients are also more susceptible to anti-IgA anaphylactoid reactions to blood/immunoglobulin/plasma product transfusions which contain IgA
The Immunoregulatory Role of Vitamin A and Retinoic Acid in the Mucosal Immune System
- Recently, it has been recognized that vitamin A and its metabolites have potent immunoregulatory activities in the mucosal immune system (Figure 3).
- Dendritic cells have been shown to produce retinoic acid, which provides yet another type of intestine-specific environmental conditioning agent for activation of T cells and B cells in the GALT and associated lymphoid tissues.
- Retinoic acid (RA), produced by gut dendritic cells or from dietary sources, not only induces addressin-associated homing receptors on T and B cells, but also provides important signals that induce differentiation and class switching of IgA-producing B cells.
- Retinoic acid also induces a subset of forkhead box P3 (FOXP3)+ regulatory T cells, which are important for maintaining immune tolerance in the gut.
- These findings show that retinoids provide important positive and negative regulatory signals to fine-tune the mucosal immune system and suggest the potential for therapeutic manipulation of the levels of retinoic acid to not only enhance immunoregulatory pathways, but to directly inhibit the generation of inflammatory T cell populations.
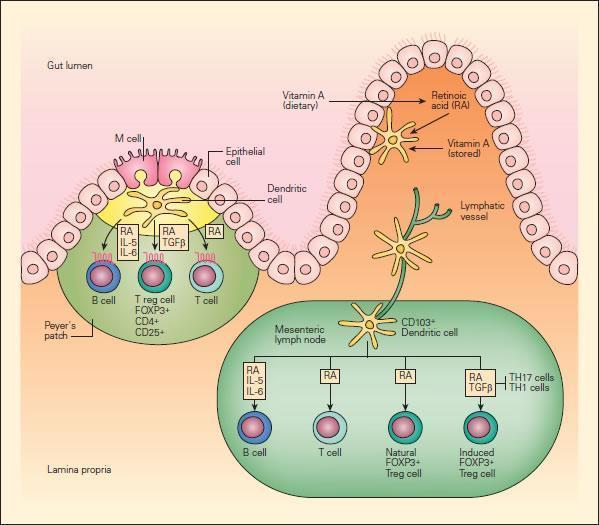
Figure 3 Schematic representation of the role of vitamin A and retinoic acid (RA) in the mucosal immune system. Intestinal DCs produce RA from stored or dietary sources of vitamin A and promote the expression of gut-homing addressin receptors by T and B lymphocytes, the peripheral generation of forkhead box P3 (FOXP3) + regulatory T (Treg) cells, and class switching to IgA. RA has an important role in these three processes. Peyer’s patch DCs and mesenteric lymph-node DCs that arrive from the intestine express enzymes that allow them to metabolize RA, perhaps from retinol carried in the serum or stored in the intestine. Alternatively, DCs may transport RA metabolized from dietary carotenoids or other vitamin A derivatives by intestinal epithelial cells to lymphoid tissues. [Reproduced with permission from Bellanti JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012]










