Introduction
- Epigenetic mechanisms have been shown to regulate many genes including those involved in inflammation and the immune response
- Epigenetic mechanisms include DNA methylation, DNA hydroxymethylation, post-translational modifications (PTMs) of histone proteins, nucleosome remodeling and regulation by non-coding RNAs
- These different mechanisms work in concert with cis and trans acting elements to drive gene expression
- Cis acting elements denote DNA sequences, which are part of gene promoters or other structural portions of a coding sequences that is required for expression
- Trans acting elements or factors are proteins that bind to cis acting sequences commanding gene or non-coding RNA expression
- Through the coordination of these acting elements, cells control DNA transcription by turning on or off coding sequences as well as non-coding functional RNAs
- There is evidence that environmental factors influence and induce long-term changes in epigenetic memory
- These include smoking, starvation, lifestyle, drugs, disease and aging.
- Consequently, the epigenetics is a bridge between environmental exposure and the more stable genetic information stored in the genome
- This could be seen as a survival advantage strategy, as it allows multicellular organisms to quickly adapt to an ever-changing environment.
- However, aberrant environmental signals, such as microbes or inflammation may induce epigenetic changes that cause dysregulation or an imbalance in immunity.
- In fact, dysregulation of epigenetic memory has been linked to pathogenic mechanisms driving the initiation and progression of a number of diseases
- Aberrant DNA methylation profiles and histone modifications have been linked to developmental defects, obesity, asthma, cancers and neurodegenerative disease.
DNA Methylation
- DNA methylation was the first epigenetic mechanism recognised and the one that is most extensively studied
- De novo methylation occurs in response to various cel- lular stressors and signal by DNA methyltransferases (Dnmt3a and Dmnt3b) which add a methyl group to position 5 of cytosine residues at a CpG site
- CpG sites are dinucleotides consisting of a cytosine and guanine (the “p” stands for the phosphodiester bond linking the 2 nucleotides) which occur throughout the genome but may be concentrated in clusters referred to as CpG islands found at important regulatory sites, such as promoter and enhancer regions
- The mechanism by which DNA methylation is associ- ated with gene silencing is still not fully understood.
Epigenetic Control of T Cells
- T cell activation and skewing, which could be viewed as a certain type of cell differentiation, is governed in great parts by epigenetic changes which insure that the clone of a T cell will retain its phenotype (Th2, Th1 or otherwise)
- Th2 skewing is triggered by simultaneous TCR and IL4 receptor activation, which leads to the phosphoryl- ation of STAT6 and expression of Th2 master regulator GATA-3 and Th2 signature cytokines, including IL-4
- Th1 differentiation is similarly triggered by simultaneous TCR and IL-12 receptor activation, phosphorylation of STAT4 and expression of Th1 master regulator TBET and Th1 signature cytokine INF-γ, with silencing of Th2 cytokines.
- In resting CD4 T cells, both IL-4 and IFN-g genes are methylated
- Upon allergenic sensitization, the IL-4 promoter in allergen-specific T cells is demethylated, the extent of which correlates with IL-4 expression
- The IL-4 locus of Th2 cells is also marked with permissive histone modifications H3K4me which are absent in Th1 or naïve T cells
- Similar modifications are found at the IFN-γ locus in Th1 cells or the IL-17 locus in Th17 cells.
- The main Th2 genes are positioned in the Th2 locus control region (LCR) on chromosome 5 which forms a chromatin hub that interacts with GATA-3 (Figure 1):
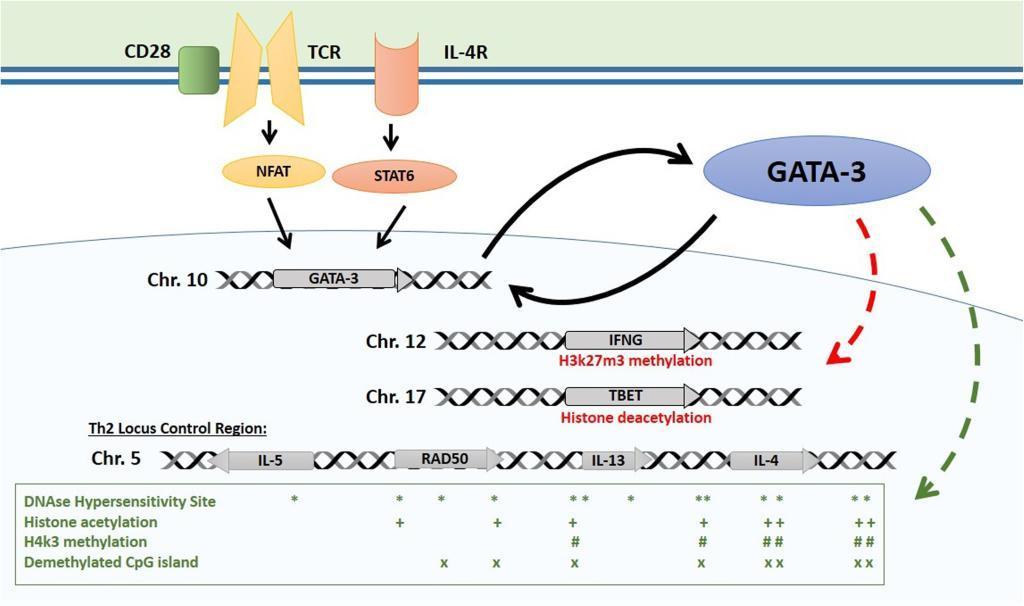
Figure 1: Epigenetic control of the Th2 locus. Master regulator GATA-3 is induced by TCR and IL-4 receptor activation and maintains its own expression with a positive feedback mechanism. GATA-3 induces repressive histone modifications at Th1 loci (TBET, IFNG). It interacts with HAT enzyme p300 with chromatin remodeling complex component Chd to induce permissive histone and chromatin changes at the Th2 LCR. Distribution of main epigenetic marks at the Th2 LCR are presented in the lower box. [Bégin, P and Nadeau KC. Epigegetic Regulation of asthma and allergic disease. Allergy, Asthma & Clinical Immunology 2014, 10:27]
- GATA-3 then interacts with HAT enzyme p300 and with chromatin remodeling complex component Chd to induce permissive histone and chromatin changes at the Th2 LCR
- The GATA-3/Chd complex also binds HDAC to repress the tbx21 locus encoding TBET, the master regulator of Th1 differentiation which activates Th1 genes and suppresses Th2 genes
- In Th1 cells, STAT4 and TBET have been shown to exert similar but inverse influence on IFN-g and Th2 genes epigenetics to pro- mote Th1 skewing
Epigenetic Control of Treg cells
- FOXP3 is the master regulator for regulatory T cells (Treg) [33] which can be divided in two subsets based on their origin: Tregs of thymic origin (tTreg), which were previously referred to as natural Tregs and peripherally- derived Treg (pTregs).
- FOXP3 expression is controlled by proximal promoter and intronic regulatory elements des- ignated as conserved non coding sequences (CNS1-3) which are highly conserved between species.
- In the thymus, tTregs are induced by TCR engagement with self-peptide major histocompatibility complex with specific strength and duration.
- The subsequent NF-κB signaling induces permissive histone modification (H3K4me1) at the CNS3 and potentially initiates chromatin remodeling in the FOXP3 locus through the c-Rel subunit
- FOXP3 induces the expression of IL-2 receptor CD25, which upon activation phosphorylates STAT5 which then binds the promoter and CNS2 independently of methylation status providing an additional positive feedback mechanism
- Besides Tregs, activated T cells also express FOXP3 upon TCR engagement. However, this expression is only transient as the CNS2 remains methylated.
- In contrast to thymic-derived Tregs, generation of Tregs from peripheral naïve T cells is favored by suboptimal TCR stimulation in the presence of TGF-β
- TGF-β promotes FOXP3 transcription in peripheral CD4 T cells through binding of SMAD3 at CNS1
- These complex interplays between T cell subsets and how gene expression is regulated by epigenetic mechanisms is a critical factor in the regulation of immune homeostatic mechanisms
- Epigenetic alterations at the level of DNA methylation have been found for several autoimmune disease (systemic lupus erythmatosus, SLE) atopy, asthma and cancers.
Immunomodulatory Agents
- Immunomodulation refers to adjusting the immune response towards a desired level
- This can mean either enhancing a sub-optimally functioning immune system, or suppressing a hyper-reactive immune system
- Immunomodulatory agents belong to three major groups (Table 1):
- group 1 (immunopotentiators) with subgroups a (nonspecific) and b (specific)
- group 2 (immunosuppressants) with similar subgroups
- group 3 (tolerance-induction)
- Immunomodulators may be beneficial, but also carry risks (e.g. general suppression using corticosteroids is beneficial in autoimmunity, but can also increase the risk of infection, cancer, and, paradoxically, autoimmunity)
- Similarly, potentiators (e.g. IFNα for chronic hepatitis B or C infection) can exacerbate autoimmunity
- Other immunomodulatory agents include TNFα inhibitors (specific immunosuppression) and growth factors (e.g. G-CSF) that stimulate production of certain immune cell populations
- Growth factors can also lead to myelodysplastic syndromes, leukemia, and other lymphoproliferative diseases
Table 1. Classification of three groups of immunomodulatory (IM) agents, with mechanisms of action, examples, and potential recipients of each
| Group | Direction of IM effect | Mechanism of action | Example of IM agents | Healthy or diseased subjects who receive immunomodulators |
|---|---|---|---|---|
| Group 1 | ||||
| Immunopotentiators | ↑ | No requirement for antigenic specificity | Adjuvants, hormones (e.g., vitamin D) | Healthy subjects receiving vaccines for prevention of infectious diseases |
| Subgroup 1a nonspecific | ||||
| Subgroup 1b specific | Require antigenic specificity | Vaccines, polyclonal antibodies, monoclonal antibodies | Antibody replacement therapy for patients with humoral immunodeficiency | |
| Group 2 | ||||
| Immunosuppressants | ↓ | Produce broad-based immunosuppression at multiple sites | Irradiation, cytotoxic drugs, glucocorticoids, immunophilins (e.g., cyclosporine), intravenous immunoglobulin (IVIG) | Subjects with autoimmune disorders, allergic diseases, and cancer |
| Subgroup 2a nonspecific | ||||
| Subgroup 2b specific | Target specific cells, cytokines, or receptors | Therapeutic polyclonal and monoclonal antibodies (e.g., anti-TNF agents), therapeutic fusion proteins (e.g., etanercept), soluble receptor constructs (e.g., anakinra), cytokines (e.g., interferons) | ||
| Group 3 | ||||
| Tolerance-inducing agents or procedures | ↓ | Directed at provoking immune tolerance by involvement of Treg cells, immunosuppressive cytokines (e.g., TGF-β, IL-10), or other mechanisms | Allergy immunotherapy (SCIT and SLIT) | Subjects with allergic disease |
Tolerance Induction
- This is the newest (possibly most specific and safest) immunomodulatory modality
- It is employed for prevention or treatment when loss of tolerance is the central pathogenic mechanisms (e.g. in allergy, asthma, and autoimmunity)
- Vaccination with self-antigens promote self-tolerance in autoimmune inbred rodent models, but translation to humans has been difficult
- Human nasal insulin vaccine to prevent type 1 diabetes mellitus shows some evidence of tolerance induction
- Human oral tolerance induction to food and inhaled agents (to treat allergy) has yielded more promising results
- Improved T-cell and cytokine knowledge will facilitate new strategies for tolerance-inducing agents and procedures
- Induction of tolerance via mucosal antigen administration is of great therapeutic interest for hypersensitivity and autoimmunity
- Symptoms of various autoimmune disorders may be diminished or reversed by induction of mucosal tolerance by oral/nasal administration of the initiating autoantigens
- MS patients undergo progressive immune-mediated myelin sheath degradation and nerve dysfunction (the mouse model suffers from experimental autoimmune encephalomyelitis [EAE])
- High oral/nasal route doses of myelin basic protein to mice suppresses myelin deterioration
- Oral administration of protein antigens in clinical trials inhibits some forms of autoimmunity (e.g. MS, RA, uveitis, diabetes)
- Mucosal immunisation for induction of tolerance may eventually provide transplant tolerance as well
Sublingual Immunotherapy (SLIT)
- Several inhaled allergen desensitisation immunotherapies exist, but subcutaneous immunotherayp (SCIT) and SLIT are relatively new and promising avenues
- Regularly administering small but gradually increasing allergen doses by the sub-lingual route can also effectively and safely increase tolerance, but requires 4-5 months of treatment, is less effective than injection immunotherapy (requiring up to 300x higher allergen doses), and response durability is unclear
- Nonetheless, SLIT may reduce the use of anti-allergic medication, and may be safer and more cost-effective than subcutaneous immunotherapy
- SLIT’s protective mechanism was initially thought to be linked to induction of IgG that prevents IgE from binding to allergens, or displaces it
- Now the role of Treg stimulation by immunotherapy is recognised, and may be the central mechanism of subcutaneous and sublingual tolerance induction










